Cell Chemical Biology ( IF 8.6 ) Pub Date : 2021-07-16 , DOI: 10.1016/j.chembiol.2021.06.007 Ying Yu Liang 1 , Smaranda Bacanu 2 , Lekshmy Sreekumar 3 , Anderson Daniel Ramos 2 , Lingyun Dai 4 , Martin Michaelis 5 , Jindrich Cinatl 6 , Takahiro Seki 7 , Yihai Cao 8 , Cynthia R Coffill 9 , David P Lane 9 , Nayana Prabhu 4 , Pär Nordlund 1
|
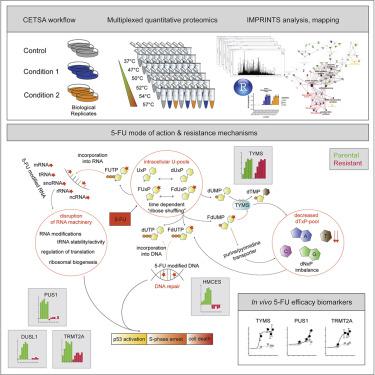
The optimal use of many cancer drugs is hampered by a lack of detailed understanding of their mechanism of action (MoA). Here, we apply a high-resolution implementation of the proteome-wide cellular thermal shift assay (CETSA) to follow protein interaction changes induced by the antimetabolite 5-fluorouracil (5-FU) and related nucleosides. We confirm anticipated effects on the known main target, thymidylate synthase (TYMS), and enzymes in pyrimidine metabolism and DNA damage pathways. However, most interaction changes we see are for proteins previously not associated with the MoA of 5-FU, including wide-ranging effects on RNA-modification and -processing pathways. Attenuated responses of specific proteins in a resistant cell model identify key components of the 5-FU MoA, where intriguingly the abrogation of TYMS inhibition is not required for cell proliferation.
中文翻译:

CETSA 相互作用蛋白质组学将特定的 RNA 修饰途径定义为基于氟尿嘧啶的癌症药物细胞毒性的关键成分
由于缺乏对其作用机制 (MoA) 的详细了解,阻碍了许多抗癌药物的最佳使用。在这里,我们应用蛋白质组范围内的细胞热迁移分析 (CETSA) 的高分辨率实施来跟踪由抗代谢物 5-氟尿嘧啶 (5-FU) 和相关核苷诱导的蛋白质相互作用变化。我们确认了对已知主要靶标胸苷酸合酶 (TYMS) 以及嘧啶代谢和 DNA 损伤途径中的酶的预期影响。然而,我们看到的大多数相互作用变化是针对以前与 5-FU 的 MoA 无关的蛋白质,包括对 RNA 修饰和加工途径的广泛影响。抗性细胞模型中特定蛋白质的减弱反应确定了 5-FU MoA 的关键成分,

























 京公网安备 11010802027423号
京公网安备 11010802027423号