当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of Spirothiazolones via Cooperative Catalysis
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-07-16 , DOI: 10.1002/adsc.202100571 Michael Franc 1 , Ivana Císařová 2 , Jan Veselý 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-07-16 , DOI: 10.1002/adsc.202100571 Michael Franc 1 , Ivana Císařová 2 , Jan Veselý 1
Affiliation
|
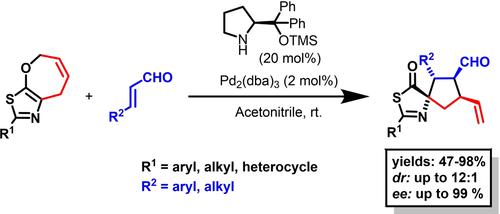
Cooperative amino- and palladium catalysis utilizing heterocyclic systems containing strongly coordinating and adsorptive sulfur atom remains an underdeveloped area. Herein, we describe an enantioselective cyclization of enals with thiazole derivatives catalyzed with the combination of achiral Pd(0) complexes and chiral secondary amines. Chiral spirocyclic thiazolones were produced in yields ranging from 41% to 98% with stereoselectivities (from 5:1 to 12:1 dr, and 95–99% ee). Moreover, the developed strategy allows access to highly substituted chiral cyclopentane derivatives by additional transformations of spirocyclic thiazolones.
中文翻译:

协同催化对映选择性合成螺噻唑酮
利用含有强配位和吸附性硫原子的杂环系统协同氨基和钯催化仍然是一个欠发达的领域。在此,我们描述了非手性 Pd(0) 配合物和手性仲胺的组合催化的烯醛与噻唑衍生物的对映选择性环化。手性螺环噻唑酮的收率范围为 41% 至 98%,具有立体选择性(5:1 至 12:1 dr,和 95-99% ee)。此外,所开发的策略允许通过螺环噻唑酮的额外转化获得高度取代的手性环戊烷衍生物。
更新日期:2021-09-21
中文翻译:

协同催化对映选择性合成螺噻唑酮
利用含有强配位和吸附性硫原子的杂环系统协同氨基和钯催化仍然是一个欠发达的领域。在此,我们描述了非手性 Pd(0) 配合物和手性仲胺的组合催化的烯醛与噻唑衍生物的对映选择性环化。手性螺环噻唑酮的收率范围为 41% 至 98%,具有立体选择性(5:1 至 12:1 dr,和 95-99% ee)。此外,所开发的策略允许通过螺环噻唑酮的额外转化获得高度取代的手性环戊烷衍生物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号