Journal of Inorganic Biochemistry ( IF 3.9 ) Pub Date : 2021-07-16 , DOI: 10.1016/j.jinorgbio.2021.111544 Tim Brückmann 1 , Jonathan Becker 1 , Christian Würtele 1 , Marcel Thomas Seuffert 1 , Dominik Heuler 1 , Klaus Müller-Buschbaum 1 , Morten Weiß 2 , Siegfried Schindler 1
|
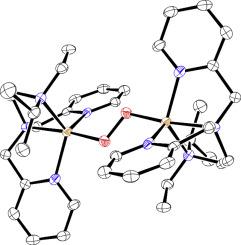
A series of copper(I) complexes with ligands derived from the tripodal ligand (2-aminoethyl)bis(2-pyridylmethyl)amine (uns-penp) have been structurally characterized and their redox chemistry analyzed by cyclic voltammetry. While the redox potentials of most of the complexes were similar their reactivity towards dioxygen was quite different. While the complex with a ferrocene derived ligand of uns-penp reacted in solution at low temperatures in a two-step reaction from the preliminary formed mononuclear end-on superoxido complex to a quite stable dinuclear peroxido complex it did not react with dioxygen in the solid state. Other complexes also did not react with dioxygen in the solid state while some showed a reversible formation to a green compound, indicating formation of an end-on superoxido complex that unfortunately so far could not be characterized. In contrast, copper complexes with the Me2uns-penp and Et-iProp-uns-penp formed dinuclear peroxido complexes in a solid-state reaction. While the reaction of dioxygen with the [Cu(Me2uns-penp]BPh4 was quite slow an instant reaction took place for [Cu(Et-iProp-uns-penp]BPh. Very unusual, it turned out that crystals of the copper(I) complex that could be structurally characterized still were crystalline when reacted with dioxygen. Therefore, it was possible to solve the structure of the corresponding dinuclear peroxido complex directly from the same batch of crystals. The crystalline structures of the copper(I) and copper(II) complex revealed that the reason for this is the fact, that the copper(I) complex is kind of preorganized for the uptake of dioxygen and does not really change in its overall structure when being oxidized.
中文翻译:

铜配合物与配体 (2-氨基乙基) 双 (2-吡啶基甲基) 胺 (uns-penp) 衍生物的表征及其对氧的反应性
一系列铜 (I) 配合物与衍生自三足配体 (2-氨基乙基) 双 (2-吡啶基甲基) 胺 (uns-penp) 的配体进行了结构表征,并通过循环伏安法分析了它们的氧化还原化学。虽然大多数配合物的氧化还原电位相似,但它们对分子氧的反应性却大不相同。虽然与二茂铁衍生的 uns-penp 配体的配合物在溶液中在低温下以两步反应从初步形成的单核末端超氧化物配合物到相当稳定的双核过氧化物配合物进行反应,但它不与固体中的分子氧反应状态。其他配合物也没有与固态的分子氧反应,而一些配合物显示出可逆的绿色化合物形成,表明形成了末端不幸的是,迄今为止无法表征的超氧化物复合物。相反,铜与 Me 2 uns-penp 和 Et-iProp-uns-penp 配合物在固态反应中形成双核过氧化物配合物。而分子氧与[Cu(Me 2 uns-penp]BPh 4[Cu(Et-iProp-uns-penp]BPh) 发生的瞬间反应非常缓慢。非常不寻常的是,可以在结构上表征的铜 (I) 配合物的晶体在与分子氧反应时仍然是结晶的。因此,可以直接从同一批晶体中解出相应的双核过氧化物配合物的结构。铜(I)络合物是一种预先组织吸收分子氧的物质,并且在被氧化时不会真正改变其整体结构。



























 京公网安备 11010802027423号
京公网安备 11010802027423号