当前位置:
X-MOL 学术
›
Aging Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Neuronal α-amylase is important for neuronal activity and glycogenolysis and reduces in presence of amyloid beta pathology
Aging Cell ( IF 7.8 ) Pub Date : 2021-07-14 , DOI: 10.1111/acel.13433 Elin Byman 1 , Isak Martinsson 2 , Henriette Haukedal 3 , 4 , Gunnar Gouras 2 , Kristine K Freude 3 , Malin Wennström 1
Aging Cell ( IF 7.8 ) Pub Date : 2021-07-14 , DOI: 10.1111/acel.13433 Elin Byman 1 , Isak Martinsson 2 , Henriette Haukedal 3 , 4 , Gunnar Gouras 2 , Kristine K Freude 3 , Malin Wennström 1
Affiliation
|
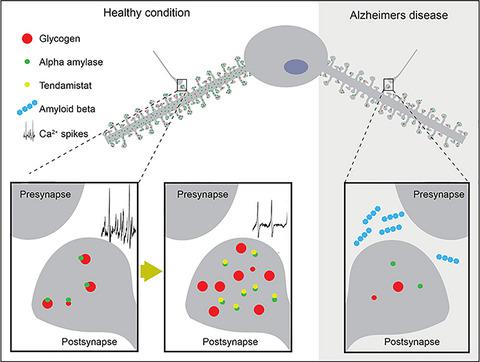
Recent studies indicate a crucial role for neuronal glycogen storage and degradation in memory formation. We have previously identified alpha-amylase (α-amylase), a glycogen degradation enzyme, located within synaptic-like structures in CA1 pyramidal neurons and shown that individuals with a high copy number variation of α-amylase perform better on the episodic memory test. We reported that neuronal α-amylase was absent in patients with Alzheimer's disease (AD) and that this loss corresponded to increased AD pathology. In the current study, we verified these findings in a larger patient cohort and determined a similar reduction in α-amylase immunoreactivity in the molecular layer of hippocampus in AD patients. Next, we demonstrated reduced α-amylase concentrations in oligomer amyloid beta 42 (Aβ42) stimulated SH-SY5Y cells and neurons derived from human-induced pluripotent stem cells (hiPSC) with PSEN1 mutation. Reduction of α-amylase production and activity, induced by siRNA and α-amylase inhibitor Tendamistat, respectively, was further shown to enhance glycogen load in SH-SY5Y cells. Both oligomer Aβ42 stimulated SH-SY5Y cells and hiPSC neurons with PSEN1 mutation showed, however, reduced load of glycogen. Finally, we demonstrate the presence of α-amylase within synapses of isolated primary neurons and show that inhibition of α-amylase activity with Tendamistat alters neuronal activity measured by calcium imaging. In view of these findings, we hypothesize that α-amylase has a glycogen degrading function within synapses, potentially important in memory formation. Hence, a loss of α-amylase, which can be induced by Aβ pathology, may in part underlie the disrupted memory formation seen in AD patients.
中文翻译:

神经元 α-淀粉酶对神经元活性和糖原分解很重要,并在存在淀粉样蛋白 β 病理学时降低
最近的研究表明神经元糖原储存和降解在记忆形成中的关键作用。我们之前已经确定了 α-淀粉酶 (α-淀粉酶),一种糖原降解酶,位于 CA1 锥体神经元的突触样结构中,并表明具有高拷贝数变异的 α-淀粉酶的个体在情景记忆测试中表现更好。我们报道阿尔茨海默病 (AD) 患者中不存在神经元 α-淀粉酶,并且这种损失对应于 AD 病理学增加。在目前的研究中,我们在更大的患者队列中验证了这些发现,并确定了 AD 患者海马分子层中 α-淀粉酶免疫反应性的类似降低。接下来,我们证明了低聚淀粉样蛋白 42(Aβ 42) 刺激来自具有 PSEN1 突变的人类诱导多能干细胞 (hiPSC) 的 SH-SY5Y 细胞和神经元。分别由 siRNA 和 α-淀粉酶抑制剂 Tendamistat 诱导的 α-淀粉酶产生和活性的降低进一步显示可增强 SH-SY5Y 细胞中的糖原负荷。低聚物 Aβ 42 然而,刺激的 SH-SY5Y 细胞和具有 PSEN1 突变的 hiPSC 神经元显示糖原负荷降低。最后,我们证明了分离的原代神经元突触中存在 α-淀粉酶,并表明用 Tendamistat 抑制 α-淀粉酶活性会改变钙成像测量的神经元活性。鉴于这些发现,我们假设 α-淀粉酶在突触内具有糖原降解功能,这在记忆形成中可能很重要。因此,可以由 Aβ 病理诱导的 α-淀粉酶丢失可能是 AD 患者记忆形成中断的部分原因。
更新日期:2021-08-19
中文翻译:

神经元 α-淀粉酶对神经元活性和糖原分解很重要,并在存在淀粉样蛋白 β 病理学时降低
最近的研究表明神经元糖原储存和降解在记忆形成中的关键作用。我们之前已经确定了 α-淀粉酶 (α-淀粉酶),一种糖原降解酶,位于 CA1 锥体神经元的突触样结构中,并表明具有高拷贝数变异的 α-淀粉酶的个体在情景记忆测试中表现更好。我们报道阿尔茨海默病 (AD) 患者中不存在神经元 α-淀粉酶,并且这种损失对应于 AD 病理学增加。在目前的研究中,我们在更大的患者队列中验证了这些发现,并确定了 AD 患者海马分子层中 α-淀粉酶免疫反应性的类似降低。接下来,我们证明了低聚淀粉样蛋白 42(Aβ 42) 刺激来自具有 PSEN1 突变的人类诱导多能干细胞 (hiPSC) 的 SH-SY5Y 细胞和神经元。分别由 siRNA 和 α-淀粉酶抑制剂 Tendamistat 诱导的 α-淀粉酶产生和活性的降低进一步显示可增强 SH-SY5Y 细胞中的糖原负荷。低聚物 Aβ 42 然而,刺激的 SH-SY5Y 细胞和具有 PSEN1 突变的 hiPSC 神经元显示糖原负荷降低。最后,我们证明了分离的原代神经元突触中存在 α-淀粉酶,并表明用 Tendamistat 抑制 α-淀粉酶活性会改变钙成像测量的神经元活性。鉴于这些发现,我们假设 α-淀粉酶在突触内具有糖原降解功能,这在记忆形成中可能很重要。因此,可以由 Aβ 病理诱导的 α-淀粉酶丢失可能是 AD 患者记忆形成中断的部分原因。


























 京公网安备 11010802027423号
京公网安备 11010802027423号