Earth and Planetary Science Letters ( IF 5.3 ) Pub Date : 2021-07-14 , DOI: 10.1016/j.epsl.2021.117071 Sae Jung Chang 1 , Ruth E. Blake 2 , Albert S. Colman 3
|
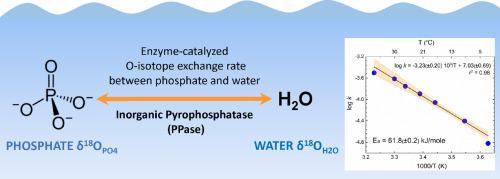
The oxygen isotope composition of phosphate (OPO4) has been widely used as (paleo)temperature and bio-signature proxies and a tracer of biogeochemical cycling of phosphorus (P). In natural aqueous systems, reactive inorganic phosphorus is dominantly present as dissolved inorganic phosphate (DIP), and reactions involving DIP are primarily carried out by microorganisms and catalyzed by enzymes. One enzyme suggested to dominate P-cycling in nature is inorganic pyrophosphatase (PPase), a ubiquitous intracellular enzyme that catalyzes oxygen (O) isotope exchange between DIP and water. Herein, we determined PPase-catalyzed O-isotope exchange rates between DIP and water over the range of typical Earth surface temperatures (3 - 37 °C) using isotope ratio mass spectrometry (IRMS). Exchange rates determined at pH 7.4 and between 3 and 37 °C, were described by first-order reaction kinetics (rate constant k = 1.51E-05 to 3.13E-04 sec−1; half-life of reaction t1/2 = 37 to 765 minutes), and strongly dependent on temperature. The temperature dependence of the exchange reaction was fitted by the Arrhenius equation, with an activation energy of 61.8 kJ/mole. The rate of PPase-catalyzed O-isotope exchange is ca. 7 - 8 orders of magnitude faster than the rate of abiotic reactions (pH 5) at 37 °C calculated by the extrapolation of high-temperature rate data. The results of this study can be used to improve the interpretation of measured O values of phosphate preserved in the rock record or during biogeochemical reactions (e.g., deconvolution of simultaneous abiotic and enzymatic/microbial processes).
中文翻译:

无机焦磷酸酶催化的磷酸盐和水之间的氧同位素交换率:对磷的生物地球化学循环的影响
磷酸盐的氧同位素组成(O PO4 ) 已被广泛用作(古)温度和生物特征代理以及磷 (P) 生物地球化学循环的示踪剂。在天然水性体系中,活性无机磷主要以溶解无机磷酸盐 (DIP) 的形式存在,涉及 DIP 的反应主要由微生物进行并由酶催化。一种在自然界中支配 P 循环的酶是无机焦磷酸酶 (PPase),它是一种普遍存在的细胞内酶,可催化 DIP 和水之间的氧 (O) 同位素交换。在此,我们 使用同位素比质谱法 (IRMS)在典型的地球表面温度 (3-37 °C)范围内确定了 PPase 催化的 O-同位素交换率。汇率在 pH 7.4 和 3 到 37 之间确定 °C,由一级反应动力学描述(速率常数k = 1.51E-05 到 3.13E-04 sec -1;反应的半衰期 t 1/2 = 37 到 765 分钟),并且强烈依赖于温度. 交换反应的温度依赖性由 Arrhenius 方程拟合,活化能为 61.8 kJ/mol。PPase 催化的 O-同位素交换的速率是大约。比 通过高温速率数据外推计算出的37 °C下的非生物反应速率 (pH 5) 快 7 - 8 个数量级。本研究的结果可用于改进测量结果的解释保存在岩石记录中或生物地球化学反应过程中的磷酸盐的 O 值(例如,同时进行的非生物和酶促/微生物过程的解卷积)。


























 京公网安备 11010802027423号
京公网安备 11010802027423号