Fluid Phase Equilibria ( IF 2.6 ) Pub Date : 2021-07-12 , DOI: 10.1016/j.fluid.2021.113135 Alireza Afsharpour 1
|
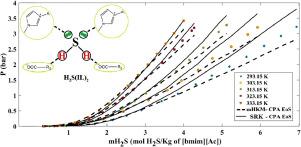
In current work, mHKM-CPA EoS together with the RETM was employed to correlate H2S absorption into five carboxylate ionic liquids, including 1-ethyl-3-methylimidazolium acetate ([emim][Ace]), 1-butyl-3-methylimidazolium acetate ([bmim][Ace]), 1-hexyl-3-methylimidazolium acetate ([hmim][Ace]), 1-ethyl-3-methylimidazolium lactate ([emim][Lac]) and 1-ethyl-3-methylimidazolium propionate ([emim][Pro]). The RETM proposes a chemical reaction between IL and H2S so that the liquid phase concentrations may be obtained by solving the model. Moreover, mHKM-CPA EoS contributes to VLE calculations. In the model, H2S considered as associating component with 4 association sites while the ILs assumed as non-self-associating compounds with one electron donor/acceptor sites.
Fourteen adjustable variables of mHKM-CPA EoS for pure components were calculated using experimental liquid density and vapor pressure data. Afterward, binary systems were correlated by applying RETM. Indeed, two nested loops calculate the liquid phase, total pressure, and vapor phase concentrations, respectively.
The parameters of the pure ILs were calculated with maximum AAD% below 0.3. Moreover, the binary results present 4.41 as the overall AAD% for 317 data points. Furthermore, a comparison between the results of the used model with those of the SRK-CPA was performed to show the capability of the mHKM-CPA EoS. As the outputs show, the model has a good ability to correlate the H2S solubility in the ILs concerning the classic SRK-CPA EoS.
中文翻译:

使用改进的 HKM 加结合 EoS 和 RETM 对某些具有羧酸根阴离子的离子液体中的 H 2 S 吸收进行建模
在目前的工作中,mHKM-CPA EoS 与 RETM 一起用于关联 H 2 S 吸收到五种羧酸盐离子液体中,包括 1-乙基-3-甲基咪唑鎓醋酸盐 ([emim][Ace])、1-丁基-3-醋酸甲基咪唑鎓 ([bmim][Ace])、1-己基-3-甲基咪唑醋酸盐 ([hmim][Ace])、1-乙基-3-甲基咪唑鎓乳酸 ([emim][Lac]) 和 1-乙基-3 -甲基咪唑丙酸([emim][Pro])。RETM 提出了 IL 和 H 2 S之间的化学反应,以便可以通过求解模型获得液相浓度。此外,mHKM-CPA EoS 有助于 VLE 计算。在模型中,H 2 S 被认为是具有 4 个缔合位点的缔合成分,而 IL 被认为是具有一个电子供体/受体位点的非自缔合化合物。
使用实验液体密度和蒸气压数据计算了纯组分的 mHKM-CPA EoS 的 14 个可调变量。之后,通过应用 RETM 将二元系统关联起来。实际上,两个嵌套循环分别计算液相、总压和气相浓度。
计算纯 IL 的参数时最大 AAD% 低于 0.3。此外,二进制结果显示 4.41 作为 317 个数据点的总体 AAD%。此外,将使用模型的结果与 SRK-CPA 的结果进行比较,以显示 mHKM-CPA EoS 的能力。如输出所示,该模型具有将 IL 中H 2 S 溶解度与经典 SRK-CPA EoS相关联的良好能力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号