当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Insight into the Therapeutic Potential of a Bicyclic Hydroxypyridone Compound 2-[(2,4-Dichlorophenyl)methyl]-7-hydroxy-1,2,3,4-tetrahydro-8H-pyrido[1,2-a]pyrazin-8-one as COMT Inhibitor in the Treatment of Parkinson's Disease: A Molecular Dynamic Simulation Approach
Chemistry & Biodiversity ( IF 2.9 ) Pub Date : 2021-07-12 , DOI: 10.1002/cbdv.202100204 Temitayo I Subair 1 , Oluwole B Akawa 1, 2 , Opeyemi S Soremekun 1 , Fisayo A Olotu 1 , Mahmoud E S Soliman 1
Chemistry & Biodiversity ( IF 2.9 ) Pub Date : 2021-07-12 , DOI: 10.1002/cbdv.202100204 Temitayo I Subair 1 , Oluwole B Akawa 1, 2 , Opeyemi S Soremekun 1 , Fisayo A Olotu 1 , Mahmoud E S Soliman 1
Affiliation
|
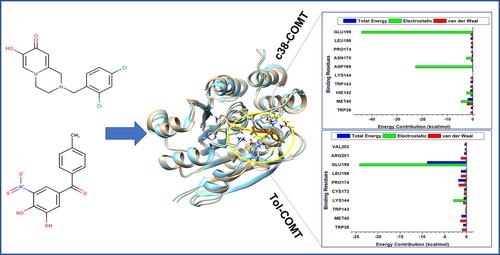
Parkinson's disease (PD) is one of the most targeted neurodegenerative diseases in clinical research. Awareness of research is due to its increasing number of affected people worldwide. The pathology of PD has been linked to several key proteins upregulation such as the catechol O-Methyltransferase (COMT). Hence, the synthesis of compounds possessing inhibitory capacity has been the frontline of research in recent years. Several compounds have been synthesized among which is the nitrocatechol. However, major limitations associated with the nitrocatechol scaffold include the inability to possess adequate CNS penetration properties and hepatic toxicity associated with the compounds. However, a series of bicyclic hydroxypyridones compounds were synthesized to evaluate their inhibitory potentials on COMT protein with compound 38 (c38) 2-[(2,4-dichlorophenyl)methyl]-7-hydroxy-1,2,3,4-tetrahydro-8H-pyrido[1,2-a]pyrazin-8-one shown to have a 40 fold increase level coverage in its IC50 over brain exposure when compared to the other synthesized compound. The molecular dynamics method was employed to understand the nature of interaction exhibited by c38. Molecular mechanics of c38 revealed a disruptive effect on the secondary structure of COMT protein. Per residue decomposition analysis revealed similar crucial residues involved in the favorable binding of c38 and tolcapone implicated its increased inhibitory capacity on COMT in preventing PD. Free binding energy (ΔGbind) of c38 further revealed the inhibitory capacity towards COMT protein in comparison to the FDA approved tolcapone. Ligand mobility analysis of both compounds showed a timewise different mobility pattern across the simulation time frame at the active site pocket of the protein connoting the different inhibitory potency exhibited by c38 and tolcapone. Findings from this study revealed optimization of c38 could facilitate the discovery of new compounds with enhanced inhibitory properties towards COMT in treating PD.
中文翻译:

深入了解双环羟基吡啶酮化合物 2-[(2,4-二氯苯基)甲基]-7-羟基-1,2,3,4-四氢-8H-吡啶并[1,2-a]吡嗪-8的治疗潜力- 作为 COMT 抑制剂治疗帕金森病:一种分子动力学模拟方法
帕金森病 (PD) 是临床研究中最具针对性的神经退行性疾病之一。研究意识是由于其在全球范围内受影响的人数不断增加。PD 的病理学与几种关键蛋白质的上调有关,例如儿茶酚 O-甲基转移酶 (COMT)。因此,合成具有抑制能力的化合物一直是近年来的研究前沿。已经合成了几种化合物,其中包括硝基儿茶酚。然而,与硝基儿茶酚支架相关的主要限制包括无法拥有足够的 CNS 渗透特性和与化合物相关的肝毒性。然而,合成了一系列双环羟基吡啶酮化合物以评估它们对 COMT 蛋白的抑制潜力,其中化合物 38 (c38) 2-[(2,H- pyrido[1,2 - a ]pyrazin-8-one与其他合成化合物相比,其 IC 50 的覆盖水平比脑暴露增加了 40 倍。采用分子动力学方法来了解 c38 所表现出的相互作用的性质。c38 的分子力学揭示了对 COMT 蛋白二级结构的破坏性影响。每残基分解分析显示,参与 c38 和 tolcapone 有利结合的类似关键残基表明其对 COMT 的抑制能力增强,以防止 PD。自由结合能(ΔG结合) 的 c38 与 FDA 批准的托卡朋相比,进一步揭示了对 COMT 蛋白的抑制能力。两种化合物的配体迁移率分析显示,在模拟时间范围内,蛋白质活性位点口袋处的迁移率模式随时间不同,这意味着 c38 和托卡朋表现出不同的抑制效力。这项研究的结果表明,c38 的优化可以促进发现在治疗 PD 中对 COMT 具有增强抑制特性的新化合物。
更新日期:2021-09-13
中文翻译:

深入了解双环羟基吡啶酮化合物 2-[(2,4-二氯苯基)甲基]-7-羟基-1,2,3,4-四氢-8H-吡啶并[1,2-a]吡嗪-8的治疗潜力- 作为 COMT 抑制剂治疗帕金森病:一种分子动力学模拟方法
帕金森病 (PD) 是临床研究中最具针对性的神经退行性疾病之一。研究意识是由于其在全球范围内受影响的人数不断增加。PD 的病理学与几种关键蛋白质的上调有关,例如儿茶酚 O-甲基转移酶 (COMT)。因此,合成具有抑制能力的化合物一直是近年来的研究前沿。已经合成了几种化合物,其中包括硝基儿茶酚。然而,与硝基儿茶酚支架相关的主要限制包括无法拥有足够的 CNS 渗透特性和与化合物相关的肝毒性。然而,合成了一系列双环羟基吡啶酮化合物以评估它们对 COMT 蛋白的抑制潜力,其中化合物 38 (c38) 2-[(2,H- pyrido[1,2 - a ]pyrazin-8-one与其他合成化合物相比,其 IC 50 的覆盖水平比脑暴露增加了 40 倍。采用分子动力学方法来了解 c38 所表现出的相互作用的性质。c38 的分子力学揭示了对 COMT 蛋白二级结构的破坏性影响。每残基分解分析显示,参与 c38 和 tolcapone 有利结合的类似关键残基表明其对 COMT 的抑制能力增强,以防止 PD。自由结合能(ΔG结合) 的 c38 与 FDA 批准的托卡朋相比,进一步揭示了对 COMT 蛋白的抑制能力。两种化合物的配体迁移率分析显示,在模拟时间范围内,蛋白质活性位点口袋处的迁移率模式随时间不同,这意味着 c38 和托卡朋表现出不同的抑制效力。这项研究的结果表明,c38 的优化可以促进发现在治疗 PD 中对 COMT 具有增强抑制特性的新化合物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号