Fluid Phase Equilibria ( IF 2.6 ) Pub Date : 2021-07-10 , DOI: 10.1016/j.fluid.2021.113169 Francisco J. Passamonti 1 , María R. Gennero de Chialvo 1 , Abel C. Chialvo 1
|
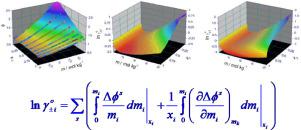
An alternative method is presented, which is independent of any physicochemical model of the electrolyte solution, for the evaluation of the mean ionic activity coefficient in molal scale, (i: 2,3), of the solutes of a ternary solution (1–2–3), starting from experimental data of the osmotic coefficient on composition ϕexp(m,xi). It is based on the dependence of ln on ϕ originally derived by H.A.C. McKay and confirmed by S.G. Canagaratna and M. Maheswaran as a particular solution of Gibbs-Duhem equation for multicomponent systems. Its application involves the integration of both ϕ and its derivative , so that it requires a very precise correlation of the dependence ϕexp(m,xi). Therefore, in order to achieve the required precision, the theoretical expression ϕ(m,xi) is developed as the sum of three contributions, (i) ϕDH: the limiting behaviour given by the Debye-Hückel equation for multicomponent systems, (ii) ΔϕDDH: the sum of the deviations to the Debye-Hückel behaviour of the corresponding binary solutions (1–2 and 1–3) and (iii) ΔϕM: a contribution of mixing effect. Finally, introducing the resulting ϕ(m,xi) into the Gibbs-Duhem equation and operating, the corresponding expression of ln (m,xi) is derived. The capacity of the ϕ(m,xi) equation to correlate the dependence ϕexp(m,xi), the methodology employed to obtain the parameters values, as well as the ln (m,xi) resulting dependence are illustrated through its application to the analysis of two ternary systems.
中文翻译:

从渗透系数数据评估三元电解质溶液活度系数的替代形式
提出了一种替代方法,它独立于电解质溶液的任何物理化学模型,用于评估摩尔尺度的平均离子活度系数, ( i : 2,3), 三元溶液 (1–2–3) 的溶质,从渗透系数的实验数据开始,组成ϕ exp ( m, x i )。它基于 ln 的依赖性on φ最初由 HAC McKay 推导出,并由 SG Canagaratna 和 M. Maheswaran 确认为多分量系统 Gibbs-Duhem 方程的特解。它的应用涉及ϕ及其导数的积分,因此,它需要的依赖的非常精确的相关性φ EXP(M,X我)。因此,为了达到所要求的精度,则理论表达式φ(M,X我)被开发为三个贡献的总和,(ⅰ)φ DH:由德拜-休克尔方程的多组分系统给定的限制的行为,( ii) Δϕ DDH:对应二元解(1-2 和 1-3)的 Debye-Hückel 行为偏差的总和和 (iii) Δϕ M:混合效应的贡献。最后,引入由此产生的ϕ ( m,x i) 代入 Gibbs-Duhem 方程并运算,ln 的对应表达式 (M,X我)被导出。所述的容量φ(M,X我)方程来的依赖相关联φ EXP(M,X我),用于获得所述参数的值的方法,以及在LN(M,X我)得到的依赖性是通过它的应用程序在两个三元系统的分析中示出。



























 京公网安备 11010802027423号
京公网安备 11010802027423号