Journal of Industrial and Engineering Chemistry ( IF 6.1 ) Pub Date : 2021-07-10 , DOI: 10.1016/j.jiec.2021.07.004 Seongsoo Kim 1 , Teayoung Lee 1 , Sanghwi Han 1 , Changha Lee 1 , Choonsoo Kim 2 , Jeyong Yoon 1, 3
|
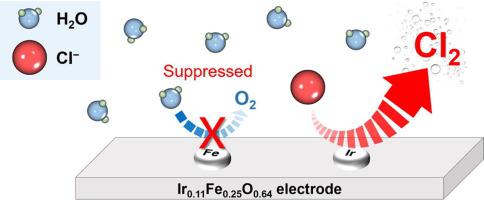
Dimensionally stable anodes (DSAs) are regarded to be optimized electrodes for electrochlorination owing to their excellent electrocatalytic activity for the chlorine evolution reaction (CER). However, in dilute chloride solutions, DSAs preferentially produce oxygen rather than chlorine because of their low overpotential for oxygen evolution reaction (OER). Considering the frequent use of electrochlorination in dilute conditions, the poor efficiency of DSAs severely limits their environmental and industrial applications. Therefore, the aim of this study is to improve the CER efficiency of IrO2 in dilute chloride solutions with the addition of Fe2O3 as a co-catalyst which has a slow reaction rate of OER. In a dilute chloride solution of 1 mM NaCl, Ir0.11Fe0.25O0.64 showed a far higher current efficiency for CER (28%) than that of IrO2 (3%). Ir0.11Fe0.25O0.64 also exhibited better current efficiency in NaCl solution of various concentrations (1 mM to 2 M) than that of IrO2. This is attributed to the synergistic effect of Fe2O3 (slow OER rate) and IrO2 (fast CER rate). Moreover, the long-term stability of Ir0.11Fe0.25O0.64 was demonstrated with tap water electrolysis for 50 days. These results suggest that Ir0.11Fe0.25O0.64 has great potential to expand the scope of application of the electrochlorination system, particularly in dilute solutions.
中文翻译:

Ir0.11Fe0.25O0.64 作为稀氯化物溶液中电解氯化的高效电极
尺寸稳定的阳极 (DSA) 被认为是用于电氯化的优化电极,因为它们对析氯反应 (CER) 具有出色的电催化活性。然而,在稀氯化物溶液中,DSA 优先产生氧而不是氯,因为它们的析氧反应 (OER) 过电位较低。考虑到在稀释条件下频繁使用电氯化,DSA 的低效率严重限制了它们的环境和工业应用。因此,本研究的目的是提高 IrO 2在稀氯化物溶液中的 CER 效率,加入 Fe 2 O 3作为 OER 反应速率缓慢的助催化剂。在 1 mM NaCl、Ir 的稀氯化物溶液中0.11 Fe 0.25 O 0.64的 CER 电流效率 (28%) 远高于 IrO 2 (3%)。Ir 0.11 Fe 0.25 O 0.64在各种浓度(1 mM 至2 M)的NaCl 溶液中也表现出比IrO 2更好的电流效率。这归因于 Fe 2 O 3(慢 OER 速率)和 IrO 2(快 CER 速率)的协同效应。此外,用自来水电解 50 天证明了Ir 0.11 Fe 0.25 O 0.64的长期稳定性。这些结果表明 Ir 0.11Fe 0.25 O 0.64具有扩大电解氯化系统应用范围的巨大潜力,特别是在稀溶液中。


























 京公网安备 11010802027423号
京公网安备 11010802027423号