Journal of Rare Earths ( IF 4.9 ) Pub Date : 2021-07-10 , DOI: 10.1016/j.jre.2021.07.002 Xiaofeng Li 1 , Yuanxing Dong 1 , Xiaohui Liu 1 , Lijun Li 1 , Yanfang Gao 1 , Zhenzhu Cao 1 , Jinrong Liu 1
|
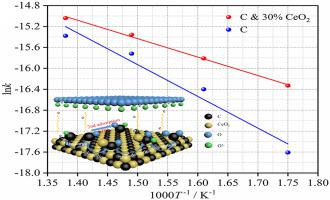
The oxidation of anode carbon fuel directly affects the electrochemical performance of molten hydroxide direct carbon fuel cell (MHDCFC). In general, the anode carbon fuel can be oxidized at high temperature, thus the direct carbon fuel cell (DCFC) can show great electrochemical performance. In this study, rare earth oxides (La2O3, CeO2, Pr6O11) were prepared by the method of precipitation. Activated carbon was prepared by pretreatment of lignite. Rare earth oxides and activated carbon were mixed as anode carbon fuel, and rare earth oxides were used to catalyze the electrochemical oxidation of anode carbon fuel. The results show that CeO2 has better electrocatalytic activity compared with La2O3 and Pr6O11 in the MHDCFC. The electrochemical test results show that the current density (at 0.4 V) increases from 81.02 to 112.90 mA/cm2 and the maximum power density increases from 34.78 to 47.05 mW/cm2 at 450 °C, when the mass fraction of CeO2 is increased from 0 to 40%. When the mass fraction of CeO2 is 30%, the current density (82.55 mA/cm2 at 0.4 V) at 400 °C is higher than that (81.02 mA/cm2 at 0.4 V) without CeO2 at 450 °C. The electrochemical oxidation mechanism of CeO2 catalyzed anode carbon fuel is discussed.
中文翻译:

稀土氧化物(La2O3、CeO2、Pr6O11)对熔融 KOH-NaOH 中活性炭电化学氧化的催化作用
阳极碳燃料的氧化直接影响熔融氢氧化物直接碳燃料电池(MHDCFC)的电化学性能。一般来说,阳极碳燃料可以在高温下被氧化,因此直接碳燃料电池(DCFC)可以表现出很好的电化学性能。本研究采用沉淀法制备稀土氧化物(La 2 O 3、CeO 2、Pr 6 O 11 )。通过褐煤预处理制备活性炭。稀土氧化物和活性炭混合作为阳极碳燃料,稀土氧化物用于催化阳极碳燃料的电化学氧化。结果表明,CeO 2在MHDCFC中与La 2 O 3和Pr 6 O 11相比具有更好的电催化活性。电化学测试结果表明,当CeO 2的质量分数为从 0 增加到 40%。当CeO 2的质量分数为30%时,400 ℃时的电流密度(0.4 V时为82.55 mA/cm 2)高于450 ℃时没有CeO 2时的电流密度(0.4 V时为81.02 mA/cm 2 )。CeO 2的电化学氧化机理讨论了催化阳极碳燃料。


























 京公网安备 11010802027423号
京公网安备 11010802027423号