Journal of Advanced Research ( IF 10.7 ) Pub Date : 2021-07-09 , DOI: 10.1016/j.jare.2021.07.003 Sandu Cibotaru 1 , Valentin Nastasa 2 , Andreea-Isabela Sandu 1 , Andra-Cristina Bostanaru 2 , Mihai Mares 2 , Luminita Marin 1
|
Introduction
Cancer is a big challenge of the 21 century, whose defeat requires efficient antitumor drugs.
Objectives
The paper aims to investigate the synergistic effect of two structural building blocks, phenothiazine and poly(ethylene glycol), towards efficient antitumor drugs.
Methods
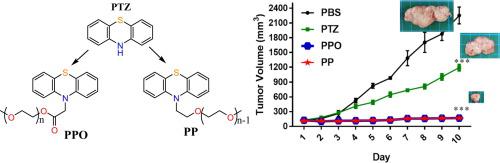
Two PEGylated phenothiazine derivatives were synthetized by attaching poly(ethylene glycol) of 550 Da to the nitrogen atom of phenothiazine by ether or ester linkage. Their antitumor activity has been investigated on five human tumour lines and a mouse tumor line as well, by determination of IC50. The in vivo toxicity was determined by measuring the LD50 in BALB/c mice by the sequential method and the in vivo antitumor potential was measured by the tumours growth test. The antitumor mechanism was investigated by complexation studies of zinc and magnesium ions characteristic to the farnesyltransferase enzyme, by studies of self-aggregation in the cells proximity and by investigation of the antitumor properties of the acid species resulted by enzymatic cleavage of the PEGylated derivatives.
Results
The two compounds showed antitumor activity, with IC50 against mouse colon carcinoma cell line comparable with that of the traditional antitumor drugs 5-Fluorouracil and doxorubicin. The phenothiazine PEGylation resulted in a significant toxicity diminishing, the LD50 in BALB/c mice increasing from 952.38 up to 1450 mg/kg, in phenothiazine equivalents. Both compounds inflicted a 92% inhibition of the tumour growth for doses much smaller than LD50. The investigation of the possible tumour inhibition mechanism suggested the nanoaggregate formation and the cleavage of ester bonds as key factors for the inhibition of cancer cell proliferation and biocompatibility improvement.
Conclusion
Phenothiazine and PEG building blocks have a synergetic effect working for both tumour growth inhibition and biocompatibility improvement. All these findings recommend the PEGylated phenothiazine derivatives as a valuable workbench for a next generation of antitumor drugs.
中文翻译:

吩噻嗪的聚乙二醇化——一种有效抗癌药物的合成途径
介绍
癌症是21世纪的一大挑战,战胜它需要高效的抗肿瘤药物。
目标
本文旨在研究吩噻嗪和聚乙二醇这两种结构单元对高效抗肿瘤药物的协同作用。
方法
通过醚或酯键将 550 Da 的聚(乙二醇)连接到吩噻嗪的氮原子上,合成了两种 PEG 化吩噻嗪衍生物。通过 IC50 的测定,已经在五种人类肿瘤系和一种小鼠肿瘤系中研究了它们的抗肿瘤活性。通过序贯法和体内法测定BALB/c小鼠体内的LD50来确定体内毒性。通过肿瘤生长试验测量抗肿瘤潜力。通过对法尼基转移酶特有的锌和镁离子的络合研究、通过研究细胞附近的自聚集以及通过对聚乙二醇化衍生物的酶促切割产生的酸性物质的抗肿瘤特性的研究,研究了抗肿瘤机制。
结果
这两种化合物显示出抗肿瘤活性,对小鼠结肠癌细胞系的IC50与传统抗肿瘤药物5-氟尿嘧啶和阿霉素相当。吩噻嗪聚乙二醇化导致毒性显着降低,BALB/c 小鼠的 LD50 从 952.38 增加到 1450 mg/kg,以吩噻嗪当量计。对于远小于 LD50 的剂量,这两种化合物都能抑制 92% 的肿瘤生长。对可能的肿瘤抑制机制的研究表明,纳米聚集体的形成和酯键的断裂是抑制癌细胞增殖和提高生物相容性的关键因素。
结论
吩噻嗪和 PEG 结构单元具有协同作用,可抑制肿瘤生长和提高生物相容性。所有这些发现都推荐聚乙二醇化吩噻嗪衍生物作为下一代抗肿瘤药物的宝贵工作台。



























 京公网安备 11010802027423号
京公网安备 11010802027423号