International Journal of Mass Spectrometry ( IF 1.8 ) Pub Date : 2021-07-09 , DOI: 10.1016/j.ijms.2021.116664 Evan Perez 1 , Irena Tatosian 1 , Amanda Bubas 1 , Anna Iacovino 1 , Susan Kline 1 , Luke Metzler 1 , Arpad Somogyi 2 , Theodore Corcovilos 3 , Michael Van Stipdonk 1
|
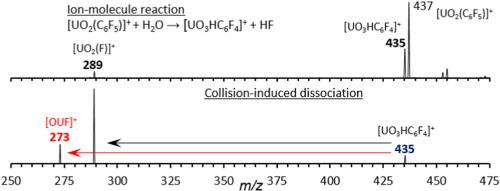
While the strong axial U O bonds confer high stability and inertness to UO22+, it has been shown that the axial oxo ligands can be eliminated or replaced in the gas-phase using collision-induced dissociation (CID) reactions. In this study, CID of the pentafluorobenzoate precursor ion [UO2(O2C–C6F5)]+ was used to produce the organo-uranyl ion [UO2(C6F5)]+ by decarboxylation. Subsequent CID of [UO2(C6F5)]+ created [UO2(F)]+ by fluoride transfer and elimination of C6F4, rather than UO2+ by elimination of pentafluorophenyl radical (as has been observed for similar species). Moreover, upon reaction of [UO2(C6F5)]+ with H2O, apparent substitution of OH for F to create [UO3HC6F4]+ is favored over hydrolysis to produce [UO2(OH)]+ and (neutral) C6F5H. Subsequent CID of [UO3HC6F4]+ generates [UO2(F)]+ and [OUF]+. When [OUF]+ is isolated to react with H2O and O2 in the ion trap, the principal product ions observed are [UO2(F)]+ and [UO2(OH)]+. Experiments conducted with labeled reagent suggest that reaction with H218O leads to exchange of the oxo ligand and incorporation of the 18O label into [OUF]+ while reaction with O2 likely creates [UO2(F)]+. Formation of [UO2(OH)]+ from [OUF]+ is the result of a cascade of reactions, with initial formation of [UO2(F)]+ by reaction with O2, followed by hydrolysis to create the hydroxide species.
O bonds confer high stability and inertness to UO22+, it has been shown that the axial oxo ligands can be eliminated or replaced in the gas-phase using collision-induced dissociation (CID) reactions. In this study, CID of the pentafluorobenzoate precursor ion [UO2(O2C–C6F5)]+ was used to produce the organo-uranyl ion [UO2(C6F5)]+ by decarboxylation. Subsequent CID of [UO2(C6F5)]+ created [UO2(F)]+ by fluoride transfer and elimination of C6F4, rather than UO2+ by elimination of pentafluorophenyl radical (as has been observed for similar species). Moreover, upon reaction of [UO2(C6F5)]+ with H2O, apparent substitution of OH for F to create [UO3HC6F4]+ is favored over hydrolysis to produce [UO2(OH)]+ and (neutral) C6F5H. Subsequent CID of [UO3HC6F4]+ generates [UO2(F)]+ and [OUF]+. When [OUF]+ is isolated to react with H2O and O2 in the ion trap, the principal product ions observed are [UO2(F)]+ and [UO2(OH)]+. Experiments conducted with labeled reagent suggest that reaction with H218O leads to exchange of the oxo ligand and incorporation of the 18O label into [OUF]+ while reaction with O2 likely creates [UO2(F)]+. Formation of [UO2(OH)]+ from [OUF]+ is the result of a cascade of reactions, with initial formation of [UO2(F)]+ by reaction with O2, followed by hydrolysis to create the hydroxide species.
中文翻译:

的创作[OUF] +使用的[UO气相反应2(C 6 ˚F 5)] +
虽然强大的轴向 U  O 键赋予 UO 2 2+高稳定性和惰性,但已经表明可以使用碰撞诱导解离 (CID) 反应在气相中消除或替换轴向氧配体。在本研究中,五氟苯甲酸盐母离子 [UO 2 (O 2 C–C 6 F 5 )] + 的CID用于通过脱羧作用产生有机铀酰离子 [UO 2 (C 6 F 5 )] +。[UO 2 (C 6 F 5 )] +创建的 [UO 2 (F)] 的后续 CID+通过氟化物转移和消除 C 6 F 4,而不是通过消除五氟苯基自由基来消除UO 2 +(如已观察到的类似物种)。此外,在 [UO 2 (C 6 F 5 )] +与 H 2 O 反应时,用 OH 表观取代 F 生成 [UO 3 HC 6 F 4 ] +比水解生成 [UO 2 (OH) ] +和(中性)C 6 F 5 H. [UO 3 HC 6 的后续 CIDF 4 ] +生成 [UO 2 (F)] +和 [OUF] +。当[OUF] +被隔离以与离子阱中的H 2 O 和O 2反应时,观察到的主要产物离子是[UO 2 (F)] +和[UO 2 (OH)] +。用标记试剂进行的实验表明,与 H 2 18 O 的反应导致氧代配体的交换和18 O 标记并入 [OUF] +而与 O 2 的反应可能会产生 [UO 2 (F)] +. 从 [OUF] +形成 [UO 2 (OH)] +是级联反应的结果,最初通过与 O 2反应形成 [UO 2 (F)] +,然后水解产生氢氧化物物质.
O 键赋予 UO 2 2+高稳定性和惰性,但已经表明可以使用碰撞诱导解离 (CID) 反应在气相中消除或替换轴向氧配体。在本研究中,五氟苯甲酸盐母离子 [UO 2 (O 2 C–C 6 F 5 )] + 的CID用于通过脱羧作用产生有机铀酰离子 [UO 2 (C 6 F 5 )] +。[UO 2 (C 6 F 5 )] +创建的 [UO 2 (F)] 的后续 CID+通过氟化物转移和消除 C 6 F 4,而不是通过消除五氟苯基自由基来消除UO 2 +(如已观察到的类似物种)。此外,在 [UO 2 (C 6 F 5 )] +与 H 2 O 反应时,用 OH 表观取代 F 生成 [UO 3 HC 6 F 4 ] +比水解生成 [UO 2 (OH) ] +和(中性)C 6 F 5 H. [UO 3 HC 6 的后续 CIDF 4 ] +生成 [UO 2 (F)] +和 [OUF] +。当[OUF] +被隔离以与离子阱中的H 2 O 和O 2反应时,观察到的主要产物离子是[UO 2 (F)] +和[UO 2 (OH)] +。用标记试剂进行的实验表明,与 H 2 18 O 的反应导致氧代配体的交换和18 O 标记并入 [OUF] +而与 O 2 的反应可能会产生 [UO 2 (F)] +. 从 [OUF] +形成 [UO 2 (OH)] +是级联反应的结果,最初通过与 O 2反应形成 [UO 2 (F)] +,然后水解产生氢氧化物物质.



























 京公网安备 11010802027423号
京公网安备 11010802027423号