当前位置:
X-MOL 学术
›
ChemPhysChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
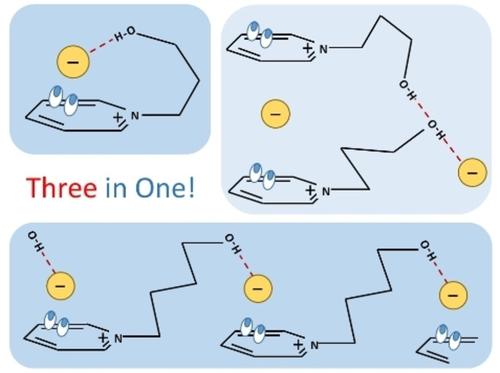
Three in One: The Versatility of Hydrogen Bonding Interaction in Halide Salts with Hydroxy-Functionalized Pyridinium Cations
ChemPhysChem ( IF 2.9 ) Pub Date : 2021-07-09 , DOI: 10.1002/cphc.202100424 Loai Al Sheakh 1 , Thomas Niemann 1 , Alexander Villinger 2 , Peter Stange 1 , Dzmitry H Zaitsau 1, 3 , Anne Strate 1, 3 , Ralf Ludwig 1, 3, 4
ChemPhysChem ( IF 2.9 ) Pub Date : 2021-07-09 , DOI: 10.1002/cphc.202100424 Loai Al Sheakh 1 , Thomas Niemann 1 , Alexander Villinger 2 , Peter Stange 1 , Dzmitry H Zaitsau 1, 3 , Anne Strate 1, 3 , Ralf Ludwig 1, 3, 4
Affiliation
|
The paradigm of supramolecular chemistry relies on the delicate balance of noncovalent forces. Here we present a systematic approach for controlling the structural versatility of halide salts by the nature of hydrogen bonding interactions. We synthesized halide salts with hydroxy-functionalized pyridinium cations [HOCnPy]+ (n=2, 3, 4) and chloride, bromide and iodide anions, which are typically used as precursor material for synthesizing ionic liquids by anion metathesis reaction. The X-ray structures of these omnium halides show two types of hydrogen bonding: ‘intra-ionic’ H-bonds, wherein the anion interacts with the hydroxy group and the positively charged ring at the same cation, and ‘inter-ionic’ H-bonds, wherein the anion also interacts with the hydroxy group and the ring system but of different cations. We show that hydrogen bonding is controllable by the length of the hydroxyalkyl chain and the interaction strength of the anion. Some molten halide salts exhibit a third type of hydrogen bonding. IR spectra reveal elusive H-bonds between the OH groups of cations, showing interaction between ions of like charge. They are formed despite the repulsive interaction between the like-charged ions and compete with the favored cation-anion H-bonds. All types of H-bonding are analyzed by quantum chemical methods and the natural bond orbital approach, emphasizing the importance of charge transfer in these interactions. For simple omnium salts, we evidenced three distinct types of hydrogen bonds: Three in one!
中文翻译:

三合一:具有羟基官能化吡啶阳离子的卤化物盐中氢键相互作用的多功能性
超分子化学的范式依赖于非共价力的微妙平衡。在这里,我们提出了一种通过氢键相互作用的性质来控制卤化物盐结构多样性的系统方法。我们合成了具有羟基官能化吡啶鎓阳离子 [HOC n Py] + ( n=2, 3, 4)和氯化物、溴化物和碘化物阴离子,它们通常用作通过阴离子复分解反应合成离子液体的前体材料。这些全卤化物的 X 射线结构显示出两种类型的氢键:“离子内”H 键,其中阴离子与羟基和带正电的环在同一阳离子处相互作用,以及“离子间”H -键,其中阴离子也与羟基和环系统相互作用,但具有不同的阳离子。我们表明氢键是由羟烷基链的长度和阴离子的相互作用强度控制的。一些熔融卤化物盐表现出第三种类型的氢键。IR 光谱揭示了阳离子的 OH 基团之间难以捉摸的 H 键,显示了相同电荷的离子之间的相互作用。尽管带相同电荷的离子之间存在排斥相互作用,但它们仍会形成,并与有利的阳离子 - 阴离子 H 键竞争。所有类型的氢键都通过量子化学方法和自然键轨道方法进行分析,强调电荷转移在这些相互作用中的重要性。对于简单的鎓盐,我们证明了三种不同类型的氢键:三合一!
更新日期:2021-09-15
中文翻译:

三合一:具有羟基官能化吡啶阳离子的卤化物盐中氢键相互作用的多功能性
超分子化学的范式依赖于非共价力的微妙平衡。在这里,我们提出了一种通过氢键相互作用的性质来控制卤化物盐结构多样性的系统方法。我们合成了具有羟基官能化吡啶鎓阳离子 [HOC n Py] + ( n=2, 3, 4)和氯化物、溴化物和碘化物阴离子,它们通常用作通过阴离子复分解反应合成离子液体的前体材料。这些全卤化物的 X 射线结构显示出两种类型的氢键:“离子内”H 键,其中阴离子与羟基和带正电的环在同一阳离子处相互作用,以及“离子间”H -键,其中阴离子也与羟基和环系统相互作用,但具有不同的阳离子。我们表明氢键是由羟烷基链的长度和阴离子的相互作用强度控制的。一些熔融卤化物盐表现出第三种类型的氢键。IR 光谱揭示了阳离子的 OH 基团之间难以捉摸的 H 键,显示了相同电荷的离子之间的相互作用。尽管带相同电荷的离子之间存在排斥相互作用,但它们仍会形成,并与有利的阳离子 - 阴离子 H 键竞争。所有类型的氢键都通过量子化学方法和自然键轨道方法进行分析,强调电荷转移在这些相互作用中的重要性。对于简单的鎓盐,我们证明了三种不同类型的氢键:三合一!



























 京公网安备 11010802027423号
京公网安备 11010802027423号