当前位置:
X-MOL 学术
›
J. Raman Spectrosc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unexpected behavior of sodium sulfate observed in experimental freezing and corrosion studies
Journal of Raman Spectroscopy ( IF 2.5 ) Pub Date : 2021-07-06 , DOI: 10.1002/jrs.6200 Ahmed Abdelmonem 1 , Nikoleta Morelová 2 , Nicolas Finck 2 , Dieter Schild 2 , Johannes Lützenkirchen 2
Journal of Raman Spectroscopy ( IF 2.5 ) Pub Date : 2021-07-06 , DOI: 10.1002/jrs.6200 Ahmed Abdelmonem 1 , Nikoleta Morelová 2 , Nicolas Finck 2 , Dieter Schild 2 , Johannes Lützenkirchen 2
Affiliation
|
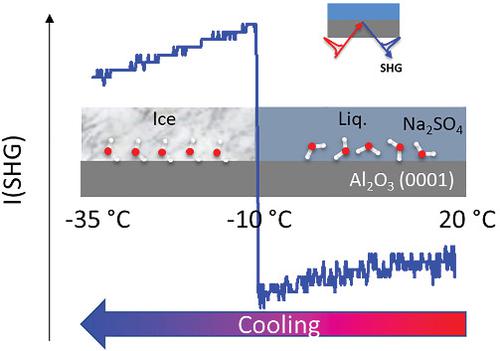
This article reports results from two distinct, originally unrelated studies that show unexpected behavior with respect to sodium sulfate solutions in contact with flat surfaces. On the one hand, we investigated immersion freezing of sulfate containing solutions (target salt was CaSO4 and Na2SO4 was used as one control) in the presence of flat sapphire-0001 crystals using second-harmonic generation (SHG) spectroscopy. Further control experiments were carried out with neat water and CaCl2. The SHG signals from CaSO4 and Na2SO4 solutions behave somewhat differently from those recorded for the two other cases, the neat water and CaCl2. The common pattern shows a decrease of the SHG signal with decreasing temperature down to about −15°C, whereupon the signal sharply drops, indicating freezing. With the Na2SO4 solution, although the signal initially follows closely the neat water curve, the trend increases sharply at about −10°C followed by a gradual decrease with further cooling. There is no plausible explanation for the distinct behavior of the Na2SO4 solution, and thermodynamic calculations do not suggest any precipitation of a sodium sulfate solid phase. At the end of the freeze–thaw cycle, the initial SHG for each system is retrieved, suggesting reversibility. On the other hand, in a separate investigation unrelated to the freezing study, it was repeatedly observed that sodium sulfate precipitates from solution on flat steel surfaces at room temperature. As in the freezing experiments, thermodynamic calculations for the bulk solution solubility of sodium sulfate indicate that precipitates should not be forming under the conditions of the studies. The crystals observed on the steel samples have snowflake shapes similar to one literature report. We conclude that Na2SO4 in the presence of flat surfaces shows unexpected behavior that should incite further detailed studies in the future to elucidate the phenomenon.
中文翻译:

在实验性冷冻和腐蚀研究中观察到硫酸钠的意外行为
本文报告了两项截然不同的、最初不相关的研究结果,这些研究显示了与平坦表面接触的硫酸钠溶液的意外行为。一方面,我们使用二次谐波发生 (SHG) 光谱研究了在平面蓝宝石 0001 晶体存在下对含硫酸盐溶液(目标盐是 CaSO 4并且使用 Na 2 SO 4作为一个对照)的浸没冷冻。进一步的对照实验是用纯水和 CaCl 2 进行的。来自 CaSO 4和 Na 2 SO 4溶液的 SHG 信号的行为与其他两种情况(纯水和 CaCl 2)记录的信号有所不同. 常见的模式显示 SHG 信号随着温度降低至 -15°C 左右而降低,随后信号急剧下降,表明冻结。对于 Na 2 SO 4溶液,虽然信号最初紧随纯水曲线,但趋势在大约 -10°C 时急剧增加,然后随着进一步冷却而逐渐减少。对于 Na 2 SO 4的独特行为没有合理的解释溶液和热力学计算表明没有任何硫酸钠固相沉淀。在冻融循环结束时,检索每个系统的初始 SHG,表明可逆性。另一方面,在与冷冻研究无关的单独调查中,反复观察到硫酸钠在室温下从扁平钢表面的溶液中沉淀出来。在冷冻实验中,硫酸钠整体溶液溶解度的热力学计算表明在研究条件下不应形成沉淀。在钢样品上观察到的晶体具有类似于一篇文献报道的雪花形状。我们得出结论,Na 2 SO 4 在平坦表面的存在下表现出意想不到的行为,应该激发未来进一步的详细研究以阐明这一现象。
更新日期:2021-09-13
中文翻译:

在实验性冷冻和腐蚀研究中观察到硫酸钠的意外行为
本文报告了两项截然不同的、最初不相关的研究结果,这些研究显示了与平坦表面接触的硫酸钠溶液的意外行为。一方面,我们使用二次谐波发生 (SHG) 光谱研究了在平面蓝宝石 0001 晶体存在下对含硫酸盐溶液(目标盐是 CaSO 4并且使用 Na 2 SO 4作为一个对照)的浸没冷冻。进一步的对照实验是用纯水和 CaCl 2 进行的。来自 CaSO 4和 Na 2 SO 4溶液的 SHG 信号的行为与其他两种情况(纯水和 CaCl 2)记录的信号有所不同. 常见的模式显示 SHG 信号随着温度降低至 -15°C 左右而降低,随后信号急剧下降,表明冻结。对于 Na 2 SO 4溶液,虽然信号最初紧随纯水曲线,但趋势在大约 -10°C 时急剧增加,然后随着进一步冷却而逐渐减少。对于 Na 2 SO 4的独特行为没有合理的解释溶液和热力学计算表明没有任何硫酸钠固相沉淀。在冻融循环结束时,检索每个系统的初始 SHG,表明可逆性。另一方面,在与冷冻研究无关的单独调查中,反复观察到硫酸钠在室温下从扁平钢表面的溶液中沉淀出来。在冷冻实验中,硫酸钠整体溶液溶解度的热力学计算表明在研究条件下不应形成沉淀。在钢样品上观察到的晶体具有类似于一篇文献报道的雪花形状。我们得出结论,Na 2 SO 4 在平坦表面的存在下表现出意想不到的行为,应该激发未来进一步的详细研究以阐明这一现象。



























 京公网安备 11010802027423号
京公网安备 11010802027423号