Synlett ( IF 2 ) Pub Date : 2021-07-06 , DOI: 10.1055/s-0040-1706053 Chuliang Gong 1 , Chen Zhang 1 , Guiyin Zhou 1 , Yao Chen 1 , Haifei Wang 1 , Xiaojun Zheng 1 , Qinglin Hou 2 , Qingxia Zhou 1
|
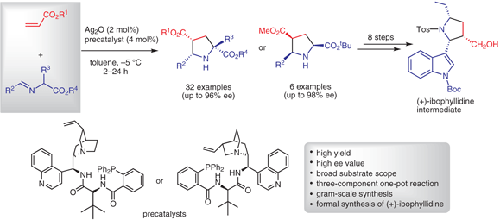
In this work, we introduced a multifunctional Ag(I)/CAAA-amidphos complex catalyzed asymmetric 1,3-dipolar cycloaddition of acrylates with α-imino esters, affording a series of 2,4,5-trisubstituted endo-pyrrolidines in good yields (up to 97%) with high enantioselectivities (up to 98% ee). Meanwhile, the catalytic system was also applied in the three-component one-pot reaction of α-imino esters formed in situ under the action of N,N′-diisopropylcarbodiimide. In addition, the gram-scale reaction was realized for the formal synthesis of (+)-ibophyllidine in eight steps.
中文翻译:

Ag(I)/CAAA-Amidphos 复合物催化丙烯酸酯的不对称 1,3-偶极环加成反应以正式合成 (+)-异叶酸
在这项工作中,我们引入了一种多功能的 Ag(I)/CAAA-酰胺磷配合物催化丙烯酸酯与 α-亚氨基酯的不对称 1,3-偶极环加成反应,以良好的收率提供了一系列 2,4,5-三取代的内-吡咯烷(高达 97%)具有高对映选择性(高达 98% ee)。同时,该催化体系也应用于N,N'-二异丙基碳二亚胺作用下原位生成的α-亚氨基酯的三组分一锅反应。此外,在八步中实现了(+)-异叶酸的正式合成的克级反应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号