当前位置:
X-MOL 学术
›
Acta Cryst. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solid-state electronegativity of atoms: new approaches
Acta Crystallographica Section B ( IF 2.684 ) Pub Date : 2021-07-05 , DOI: 10.1107/s2052520621004704 Stepan S. Batsanov , Andrei S. Batsanov
Acta Crystallographica Section B ( IF 2.684 ) Pub Date : 2021-07-05 , DOI: 10.1107/s2052520621004704 Stepan S. Batsanov , Andrei S. Batsanov
|
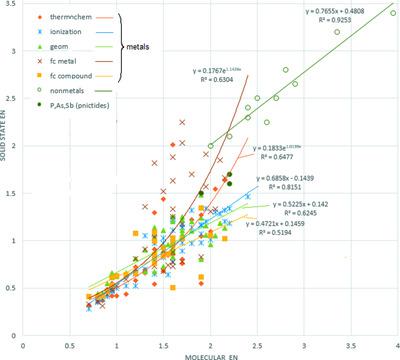
Electronegativities (EN) of 65 elements (H to Bi, except lanthanides and noble gases, plus U and Th) in solids were derived from various observed parameters, namely, bond energies in solids, structural geometry, work functions and force constants, yielding a set of internally consistent values. The solid-state electronegativities are generally lower than the conventional (`molecular') values, due to different coordination numbers and electronic structure in a solid versus a molecule; the decrease is stronger for metals than for non-metals, hence binary compounds have a wider EN difference and higher bond polarity (ionicity) in the solid than in the molecular (gaseous) state. Under high pressure, the ENs of metals increase and those of non-metals decrease, the binary solid becomes less polar and can ultimately dissociate into elements.
中文翻译:

原子的固态电负性:新方法
固体中 65 种元素(H 到 Bi,除了镧系元素和惰性气体,加上 U 和 Th)的电负性 (EN) 来自各种观察到的参数,即固体中的键能、结构几何、功函数和力常数,产生一个一组内部一致的值。由于固体与分子的配位数和电子结构不同,固态电负性通常低于常规(“分子”)值;金属比非金属的下降幅度更大,因此二元化合物在固体中比在分子(气态)状态下具有更宽的 EN 差异和更高的键极性(离子性)。在高压下,金属的 EN 增加,非金属的 EN 减少,二元固体的极性降低,最终可以分解成元素。
更新日期:2021-08-05
中文翻译:

原子的固态电负性:新方法
固体中 65 种元素(H 到 Bi,除了镧系元素和惰性气体,加上 U 和 Th)的电负性 (EN) 来自各种观察到的参数,即固体中的键能、结构几何、功函数和力常数,产生一个一组内部一致的值。由于固体与分子的配位数和电子结构不同,固态电负性通常低于常规(“分子”)值;金属比非金属的下降幅度更大,因此二元化合物在固体中比在分子(气态)状态下具有更宽的 EN 差异和更高的键极性(离子性)。在高压下,金属的 EN 增加,非金属的 EN 减少,二元固体的极性降低,最终可以分解成元素。



























 京公网安备 11010802027423号
京公网安备 11010802027423号