当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Towards integrated production of an influenza A vaccine candidate with MDCK suspension cells
Biotechnology and Bioengineering ( IF 3.8 ) Pub Date : 2021-07-05 , DOI: 10.1002/bit.27876 Thomas Bissinger 1 , Yixiao Wu 1, 2 , Pavel Marichal-Gallardo 1 , Dietmar Riedel 3 , Xuping Liu 4 , Yvonne Genzel 1 , Wen-Song Tan 2, 4 , Udo Reichl 1, 5
Biotechnology and Bioengineering ( IF 3.8 ) Pub Date : 2021-07-05 , DOI: 10.1002/bit.27876 Thomas Bissinger 1 , Yixiao Wu 1, 2 , Pavel Marichal-Gallardo 1 , Dietmar Riedel 3 , Xuping Liu 4 , Yvonne Genzel 1 , Wen-Song Tan 2, 4 , Udo Reichl 1, 5
Affiliation
|
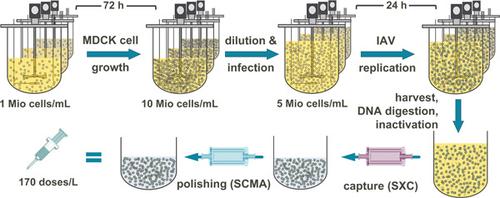
Seasonal influenza epidemics occur both in northern and southern hemispheres every year. Despite the differences in influenza virus surface antigens and virulence of seasonal subtypes, manufacturers are well-adapted to respond to this periodical vaccine demand. Due to decades of influenza virus research, the development of new influenza vaccines is relatively straight forward. In similarity with the ongoing coronavirus disease 2019 pandemic, vaccine manufacturing is a major bottleneck for a rapid supply of the billions of doses required worldwide. In particular, egg-based vaccine production would be difficult to schedule and shortages of other egg-based vaccines with high demands also have to be anticipated. Cell culture-based production systems enable the manufacturing of large amounts of vaccines within a short time frame and expand significantly our options to respond to pandemics and emerging viral diseases. In this study, we present an integrated process for the production of inactivated influenza A virus vaccines based on a Madin–Darby Canine Kidney (MDCK) suspension cell line cultivated in a chemically defined medium. Very high titers of 3.6 log10(HAU/100 µl) were achieved using fast-growing MDCK cells at concentrations up to 9.5 × 106 cells/ml infected with influenza A/PR/8/34 H1N1 virus in 1 L stirred tank bioreactors. A combination of membrane-based steric-exclusion chromatography followed by pseudo-affinity chromatography with a sulfated cellulose membrane adsorber enabled full recovery for the virus capture step and up to 80% recovery for the virus polishing step. Purified virus particles showed a homogenous size distribution with a mean diameter of 80 nm. Based on a monovalent dose of 15 µg hemagglutinin (single-radial immunodiffusion assay), the level of total protein and host cell DNA was 58 µg and 10 ng, respectively. Furthermore, all process steps can be fully scaled up to industrial quantities for commercial manufacturing of either seasonal or pandemic influenza virus vaccines. Fast production of up to 300 vaccine doses per liter within 4–5 days makes this process competitive not only to other cell-based processes but to egg-based processes as well.
中文翻译:

使用 MDCK 悬浮细胞集成生产甲型流感疫苗候选物
每年在北半球和南半球都会发生季节性流感流行。尽管流感病毒表面抗原和季节性亚型的毒力存在差异,但制造商能够很好地适应这种周期性的疫苗需求。由于数十年的流感病毒研究,新型流感疫苗的开发相对简单。与持续的 2019 年冠状病毒病大流行相似,疫苗制造是快速供应全球所需的数十亿剂疫苗的主要瓶颈。特别是,鸡蛋疫苗的生产将难以安排,其他高需求的鸡蛋疫苗也将出现短缺。基于细胞培养的生产系统能够在短时间内生产大量疫苗,并显着扩大我们应对流行病和新出现的病毒性疾病的选择。在本研究中,我们提出了一种基于在化学成分确定的培养基中培养的 Madin-Darby Canine Kidney (MDCK) 悬浮细胞系生产灭活甲型流感病毒疫苗的综合过程。3.6 log 的非常高的滴度使用浓度高达 9.5 × 10 6 的快速生长的 MDCK 细胞获得10 (HAU/100 µl)在 1 L 搅拌罐生物反应器中感染甲型流感/PR/8/34 H1N1 病毒的细胞/毫升。将基于膜的空间排阻层析和伪亲和层析与硫酸化纤维素膜吸附剂相结合,使病毒捕获步骤的完全回收率和病毒精制步骤的回收率达到 80%。纯化的病毒颗粒显示出均匀的尺寸分布,平均直径为 80 nm。基于 15 µg 血凝素的单价剂量(单径向免疫扩散试验),总蛋白和宿主细胞 DNA 的水平分别为 58 µg 和 10 ng。此外,所有工艺步骤都可以完全扩大到工业规模,用于季节性或大流行性流感病毒疫苗的商业生产。
更新日期:2021-09-12
中文翻译:

使用 MDCK 悬浮细胞集成生产甲型流感疫苗候选物
每年在北半球和南半球都会发生季节性流感流行。尽管流感病毒表面抗原和季节性亚型的毒力存在差异,但制造商能够很好地适应这种周期性的疫苗需求。由于数十年的流感病毒研究,新型流感疫苗的开发相对简单。与持续的 2019 年冠状病毒病大流行相似,疫苗制造是快速供应全球所需的数十亿剂疫苗的主要瓶颈。特别是,鸡蛋疫苗的生产将难以安排,其他高需求的鸡蛋疫苗也将出现短缺。基于细胞培养的生产系统能够在短时间内生产大量疫苗,并显着扩大我们应对流行病和新出现的病毒性疾病的选择。在本研究中,我们提出了一种基于在化学成分确定的培养基中培养的 Madin-Darby Canine Kidney (MDCK) 悬浮细胞系生产灭活甲型流感病毒疫苗的综合过程。3.6 log 的非常高的滴度使用浓度高达 9.5 × 10 6 的快速生长的 MDCK 细胞获得10 (HAU/100 µl)在 1 L 搅拌罐生物反应器中感染甲型流感/PR/8/34 H1N1 病毒的细胞/毫升。将基于膜的空间排阻层析和伪亲和层析与硫酸化纤维素膜吸附剂相结合,使病毒捕获步骤的完全回收率和病毒精制步骤的回收率达到 80%。纯化的病毒颗粒显示出均匀的尺寸分布,平均直径为 80 nm。基于 15 µg 血凝素的单价剂量(单径向免疫扩散试验),总蛋白和宿主细胞 DNA 的水平分别为 58 µg 和 10 ng。此外,所有工艺步骤都可以完全扩大到工业规模,用于季节性或大流行性流感病毒疫苗的商业生产。


























 京公网安备 11010802027423号
京公网安备 11010802027423号