当前位置:
X-MOL 学术
›
Mol. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Autophosphorylation of the KaiC-like protein ArlH inhibits oligomerization and interaction with ArlI, the motor ATPase of the archaellum
Molecular Microbiology ( IF 3.6 ) Pub Date : 2021-07-05 , DOI: 10.1111/mmi.14781 J Nuno de Sousa Machado 1, 2 , Leonie Vollmar 2, 3 , Julia Schimpf 2, 3 , Paushali Chaudhury 1 , Rashmi Kumariya 1 , Chris van der Does 1 , Thorsten Hugel 3 , Sonja-Verena Albers 1
Molecular Microbiology ( IF 3.6 ) Pub Date : 2021-07-05 , DOI: 10.1111/mmi.14781 J Nuno de Sousa Machado 1, 2 , Leonie Vollmar 2, 3 , Julia Schimpf 2, 3 , Paushali Chaudhury 1 , Rashmi Kumariya 1 , Chris van der Does 1 , Thorsten Hugel 3 , Sonja-Verena Albers 1
Affiliation
|
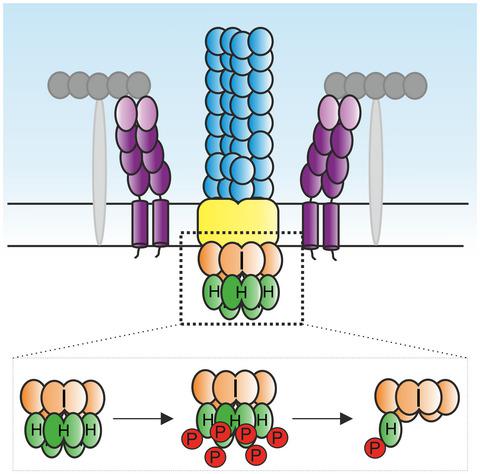
Motile archaea are propelled by the archaellum, whose motor complex consists of the membrane protein ArlJ, the ATPase ArlI, and the ATP-binding protein ArlH. Despite its essential function and the existence of structural and biochemical data on ArlH, the role of ArlH in archaellum assembly and function remains elusive. ArlH is a structural homolog of KaiC, the central component of the cyanobacterial circadian clock. Since autophosphorylation and dephosphorylation of KaiC are central properties for the function of KaiC, we asked whether autophosphorylation is also a property of ArlH proteins. We observed that both ArlH from the euryarchaeon Pyrococcus furiosus (PfArlH) and from the crenarchaeon Sulfolobus acidocaldarius (SaArlH) have autophosphorylation activity. Using a combination of single-molecule fluorescence measurements and biochemical assays, we show that autophosphorylation of ArlH is closely linked to its oligomeric state when bound to hexameric ArlI. These experiments also strongly suggest that ArlH is a hexamer in its ArlI-bound state. Mutagenesis of the putative catalytic residue (Glu-57 in SaArlH) in ArlH results in a reduced autophosphorylation activity and abolished archaellation and motility in S. acidocaldarius, indicating that optimum phosphorylation activity of ArlH is essential for archaellation and motility.
中文翻译:

KaiC样蛋白ArlH的自磷酸化抑制寡聚化和与ArlI的相互作用,Arli是古细菌的运动ATP酶
运动古生菌由古细菌推动,其运动复合物由膜蛋白 ArlJ、ATP 酶 ArlI 和 ATP 结合蛋白 ArlH 组成。尽管 ArlH 具有重要功能并且存在结构和生化数据,但 ArlH 在古细菌组装和功能中的作用仍然难以捉摸。ArlH 是 KaiC 的结构同源物,KaiC 是蓝藻生物钟的中心组成部分。由于 KaiC 的自磷酸化和去磷酸化是 KaiC 功能的核心特性,我们询问自磷酸化是否也是 ArlH 蛋白的特性。我们观察到来自 euryarchaeon Pyrococcus furiosus ( Pf ArlH) 和来自 crenarchaeon Sulfolobus acidocaldarius ( SaArlH)具有自磷酸化活性。我们结合使用单分子荧光测量和生化测定,表明 ArlH 的自磷酸化与其六聚体 ArlI 结合时的寡聚状态密切相关。这些实验还强烈表明 ArlH 是 ArlI 结合状态的六聚体。ArlH中推定的催化残基(Sa ArlH 中的 Glu-57)的诱变导致S. acidocaldarius中的自磷酸化活性降低并消除了螺旋化和运动,表明 ArlH 的最佳磷酸化活性对于螺旋化和运动至关重要。
更新日期:2021-07-05
中文翻译:

KaiC样蛋白ArlH的自磷酸化抑制寡聚化和与ArlI的相互作用,Arli是古细菌的运动ATP酶
运动古生菌由古细菌推动,其运动复合物由膜蛋白 ArlJ、ATP 酶 ArlI 和 ATP 结合蛋白 ArlH 组成。尽管 ArlH 具有重要功能并且存在结构和生化数据,但 ArlH 在古细菌组装和功能中的作用仍然难以捉摸。ArlH 是 KaiC 的结构同源物,KaiC 是蓝藻生物钟的中心组成部分。由于 KaiC 的自磷酸化和去磷酸化是 KaiC 功能的核心特性,我们询问自磷酸化是否也是 ArlH 蛋白的特性。我们观察到来自 euryarchaeon Pyrococcus furiosus ( Pf ArlH) 和来自 crenarchaeon Sulfolobus acidocaldarius ( SaArlH)具有自磷酸化活性。我们结合使用单分子荧光测量和生化测定,表明 ArlH 的自磷酸化与其六聚体 ArlI 结合时的寡聚状态密切相关。这些实验还强烈表明 ArlH 是 ArlI 结合状态的六聚体。ArlH中推定的催化残基(Sa ArlH 中的 Glu-57)的诱变导致S. acidocaldarius中的自磷酸化活性降低并消除了螺旋化和运动,表明 ArlH 的最佳磷酸化活性对于螺旋化和运动至关重要。



























 京公网安备 11010802027423号
京公网安备 11010802027423号