当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
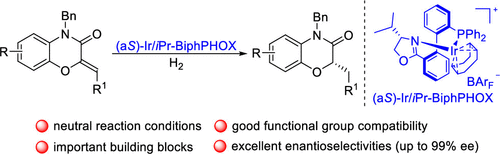
Synthesis of Chiral 2-Substituted 1,4-Benzoxazin-3-ones via Iridium-Catalyzed Enantioselective Hydrogenation of Benzoxazinones
Organic Letters ( IF 5.2 ) Pub Date : 2021-07-02 , DOI: 10.1021/acs.orglett.1c01701 Yu Nie 1 , Jing Li 1 , Jun Yan 1 , Qianjia Yuan 1 , Wanbin Zhang 1, 2
Organic Letters ( IF 5.2 ) Pub Date : 2021-07-02 , DOI: 10.1021/acs.orglett.1c01701 Yu Nie 1 , Jing Li 1 , Jun Yan 1 , Qianjia Yuan 1 , Wanbin Zhang 1, 2
Affiliation
|
An efficient iridium-catalyzed enantioselective hydrogenation of 2-alkylidene 1,4-benzoxazin-3-ones using our developed iPr-BiphPHOX as a ligand is reported. This method showed good functional group compatibility and delivered the corresponding reduced products in excellent yields (up to 99%) with excellent enantioselectivities (up to 99% ee). The reaction proceeded very well on a gram scale with low catalyst loadings (0.1 mol %), providing the product with no erosion in enantioselectivity. Additionally, three bioactive molecules can be easily obtained from the reduced products.
中文翻译:

通过铱催化苯并恶嗪酮的对映选择性氢化合成手性 2-取代的 1,4-苯并恶嗪-3-酮
报道了使用我们开发的i Pr-BiphPHOX 作为配体对 2-亚烷基 1,4-苯并恶嗪-3-酮进行有效的铱催化对映选择性氢化。该方法显示出良好的官能团兼容性,并以优异的产率(高达 99%)和优异的对映选择性(高达 99% ee)提供相应的还原产物。该反应在克级规模下以低催化剂负载量(0.1 mol%)进行得非常好,提供了对映选择性没有侵蚀的产物。此外,从还原产物中可以很容易地获得三种生物活性分子。
更新日期:2021-07-16
中文翻译:

通过铱催化苯并恶嗪酮的对映选择性氢化合成手性 2-取代的 1,4-苯并恶嗪-3-酮
报道了使用我们开发的i Pr-BiphPHOX 作为配体对 2-亚烷基 1,4-苯并恶嗪-3-酮进行有效的铱催化对映选择性氢化。该方法显示出良好的官能团兼容性,并以优异的产率(高达 99%)和优异的对映选择性(高达 99% ee)提供相应的还原产物。该反应在克级规模下以低催化剂负载量(0.1 mol%)进行得非常好,提供了对映选择性没有侵蚀的产物。此外,从还原产物中可以很容易地获得三种生物活性分子。


























 京公网安备 11010802027423号
京公网安备 11010802027423号