当前位置:
X-MOL 学术
›
Brain Pathol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dysregulated protein phosphorylation: A determining condition in the continuum of brain aging and Alzheimer's disease
Brain Pathology ( IF 6.4 ) Pub Date : 2021-07-04 , DOI: 10.1111/bpa.12996 Isidro Ferrer 1, 2, 3 , Pol Andrés-Benito 1, 2, 3 , Karina Ausín 4 , Reinald Pamplona 5 , José Antonio Del Rio 6, 7 , Joaquín Fernández-Irigoyen 4 , Enrique Santamaría 4
Brain Pathology ( IF 6.4 ) Pub Date : 2021-07-04 , DOI: 10.1111/bpa.12996 Isidro Ferrer 1, 2, 3 , Pol Andrés-Benito 1, 2, 3 , Karina Ausín 4 , Reinald Pamplona 5 , José Antonio Del Rio 6, 7 , Joaquín Fernández-Irigoyen 4 , Enrique Santamaría 4
Affiliation
|
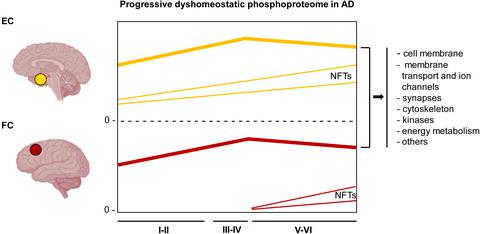
Tau hyperphosphorylation is the first step of neurofibrillary tangle (NFT) formation. In the present study, samples of the entorhinal cortex (EC) and frontal cortex area 8 (FC) of cases with NFT pathology classified as stages I–II, III–IV, and V–VI without comorbidities, and of middle-aged (MA) individuals with no NFT pathology, were analyzed by conventional label-free and SWATH-MS (sequential window acquisition of all theoretical fragment ion spectra mass spectrometry) to assess the (phospho)proteomes. The total number of identified dysregulated phosphoproteins was 214 in the EC, 65 of which were dysregulated at the first stages (I–II) of NFT pathology; 167 phosphoproteins were dysregulated in the FC, 81 of them at stages I–II of NFT pathology. A large percentage of dysregulated phosphoproteins were identified in the two regions and at different stages of NFT progression. The main group of dysregulated phosphoproteins was made up of components of the membranes, cytoskeleton, synapses, proteins linked to membrane transport and ion channels, and kinases. The present results show abnormal phosphorylation of proteins at the first stages of NFT pathology in the elderly (in individuals clinically considered representative of normal aging) and sporadic Alzheimer's disease (sAD). Dysregulated protein phosphorylation in the FC precedes the formation of NFTs and SPs. The most active period of dysregulated phosphorylation is at stages III–IV when a subpopulation of individuals might be clinically categorized as suffering from mild cognitive impairment which is a preceding determinant stage in the progression to dementia. Altered phosphorylation of selected proteins, carried out by activation of several kinases, may alter membrane and cytoskeletal functions, among them synaptic transmission and membrane/cytoskeleton signaling. Besides their implications in sAD, the present observations suggest a molecular substrate for “benign” cognitive deterioration in “normal” brain aging.
中文翻译:

蛋白质磷酸化失调:脑衰老和阿尔茨海默病连续过程中的决定性条件
Tau 过度磷酸化是神经原纤维缠结 (NFT) 形成的第一步。在本研究中,NFT 病理分为 I-II、III-IV 和 V-VI 期且无合并症的病例的内嗅皮层 (EC) 和额叶皮层区域 8 (FC) 样本,以及中年 ( MA) 没有 NFT 病理学的个体通过常规无标记和 SWATH-MS(所有理论碎片离子光谱质谱法的顺序窗口采集)进行分析,以评估(磷酸)蛋白质组。EC 中鉴定的失调磷蛋白总数为 214,其中 65 个在 NFT 病理学的第一阶段(I-II)失调;167 种磷蛋白在 FC 中失调,其中 81 种处于 NFT 病理学的 I-II 阶段。在这两个区域和 NFT 进展的不同阶段发现了很大比例的失调磷蛋白。主要的失调磷蛋白组由膜、细胞骨架、突触、与膜转运和离子通道相关的蛋白质以及激酶的成分组成。目前的结果显示老年人(在临床上被认为代表正常衰老的个体)和散发性阿尔茨海默病 (sAD) 的 NFT 病理学的第一阶段蛋白质的异常磷酸化。FC 中蛋白质磷酸化失调先于 NFT 和 SP 的形成。磷酸化失调最活跃的时期是在 III-IV 阶段,此时个体亚群可能在临床上被归类为患有轻度认知障碍,这是发展为痴呆的前一个决定性阶段。通过激活几种激酶来改变所选蛋白质的磷酸化可能会改变膜和细胞骨架功能,其中包括突触传递和膜/细胞骨架信号传导。除了它们对 sAD 的影响外,目前的观察结果表明,在“正常”脑老化中“良性”认知退化的分子基质。
更新日期:2021-07-04
中文翻译:

蛋白质磷酸化失调:脑衰老和阿尔茨海默病连续过程中的决定性条件
Tau 过度磷酸化是神经原纤维缠结 (NFT) 形成的第一步。在本研究中,NFT 病理分为 I-II、III-IV 和 V-VI 期且无合并症的病例的内嗅皮层 (EC) 和额叶皮层区域 8 (FC) 样本,以及中年 ( MA) 没有 NFT 病理学的个体通过常规无标记和 SWATH-MS(所有理论碎片离子光谱质谱法的顺序窗口采集)进行分析,以评估(磷酸)蛋白质组。EC 中鉴定的失调磷蛋白总数为 214,其中 65 个在 NFT 病理学的第一阶段(I-II)失调;167 种磷蛋白在 FC 中失调,其中 81 种处于 NFT 病理学的 I-II 阶段。在这两个区域和 NFT 进展的不同阶段发现了很大比例的失调磷蛋白。主要的失调磷蛋白组由膜、细胞骨架、突触、与膜转运和离子通道相关的蛋白质以及激酶的成分组成。目前的结果显示老年人(在临床上被认为代表正常衰老的个体)和散发性阿尔茨海默病 (sAD) 的 NFT 病理学的第一阶段蛋白质的异常磷酸化。FC 中蛋白质磷酸化失调先于 NFT 和 SP 的形成。磷酸化失调最活跃的时期是在 III-IV 阶段,此时个体亚群可能在临床上被归类为患有轻度认知障碍,这是发展为痴呆的前一个决定性阶段。通过激活几种激酶来改变所选蛋白质的磷酸化可能会改变膜和细胞骨架功能,其中包括突触传递和膜/细胞骨架信号传导。除了它们对 sAD 的影响外,目前的观察结果表明,在“正常”脑老化中“良性”认知退化的分子基质。



























 京公网安备 11010802027423号
京公网安备 11010802027423号