Journal of Allergy and Clinical Immunology ( IF 14.2 ) Pub Date : 2021-07-02 , DOI: 10.1016/j.jaci.2021.06.024 Janet Chou 1 , Craig D Platt 1 , Saddiq Habiballah 1 , Alan A Nguyen 1 , Megan Elkins 1 , Sabrina Weeks 1 , Zachary Peters 1 , Megan Day-Lewis 1 , Tanya Novak 2 , Myriam Armant 3 , Lucinda Williams 4 , Shira Rockowitz 5 , Piotr Sliz 5 , David A Williams 6 , Adrienne G Randolph 2 , Raif S Geha 1 ,
|
Background
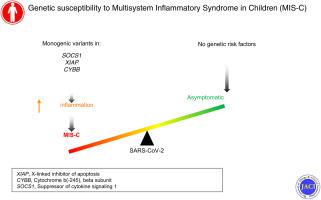
Multisystem inflammatory syndrome in children (MIS-C) is a pediatric complication of severe acute respiratory syndrome coronavirus 2 infection that is characterized by multiorgan inflammation and frequently by cardiovascular dysfunction. It occurs predominantly in otherwise healthy children. We previously reported haploinsufficiency of suppressor of cytokine signaling 1 (SOCS1), a negative regulator of type I and II interferons, as a genetic risk factor for MIS-C.
Objectives
We aimed to identify additional genetic mechanisms underlying susceptibility to severe acute respiratory syndrome coronavirus 2–associated MIS-C.
Methods
In a single-center, prospective cohort study, whole exome sequencing was performed on patients with MIS-C. The impact of candidate variants was tested by using patients’ PBMCs obtained at least 7 months after recovery.
Results
We enrolled 18 patients with MIS-C (median age = 8 years; interquartile range = 5-12.25 years), of whom 89% had no conditions other than obesity. In 2 boys with no significant infection history, we identified and validated hemizygous deleterious defects in XIAP, encoding X-linked inhibitor of apoptosis, and CYBB, encoding cytochrome b-245, beta subunit. Including the previously reported SOCS1 haploinsufficiency, a genetic diagnosis was identified in 3 of 18 patients (17%). In contrast to patients with mild COVID-19, patients with defects in SOCS1, XIAP, or CYBB exhibit an inflammatory immune cell transcriptome with enrichment of differentially expressed genes in pathways downstream of IL-18, oncostatin M, and nuclear factor κB, even after recovery.
Conclusions
Although inflammatory disorders are rare in the general population, our cohort of patients with MIS-C was enriched for monogenic susceptibility to inflammation. Our results support the use of next-generation sequencing in previously healthy children who develop MIS-C.
中文翻译:

儿童多系统炎症综合征遗传易感性的机制(MIS-C)
背景
儿童多系统炎症综合征 (MIS-C) 是严重急性呼吸综合征冠状病毒 2 感染的一种儿科并发症,其特征是多器官炎症和心血管功能障碍。它主要发生在其他方面健康的儿童中。我们之前报道过细胞因子信号传导抑制因子 1 (SOCS1)(I 型和 II 型干扰素的负调节因子)的单倍体不足,是 MIS-C 的遗传危险因素。
目标
我们的目的是确定与严重急性呼吸综合征冠状病毒 2 相关的 MIS-C 易感性的其他遗传机制。
方法
在一项单中心前瞻性队列研究中,对 MIS-C 患者进行了全外显子组测序。通过使用患者康复后至少 7 个月获得的 PBMC 来测试候选变异的影响。
结果
我们招募了 18 名 MIS-C 患者(中位年龄 = 8 岁;四分位数范围 = 5-12.25 岁),其中 89% 除肥胖外没有其他疾病。在 2 名没有明显感染史的男孩中,我们鉴定并验证了XIAP(编码 X 连锁凋亡抑制剂)和CYBB(编码细胞色素 b-245、β 亚基)的半合子有害缺陷。包括之前报道的 SOCS1 单倍体不足在内,18 名患者中有 3 名 (17%) 被确诊为基因诊断。与轻度 COVID-19 患者相比,SOCS1、XIAP 或 CYBB 缺陷的患者表现出炎症免疫细胞转录组,其中 IL-18、制瘤素 M 和核因子 κB 下游通路中的差异表达基因富集,即使在恢复。
结论
尽管炎症性疾病在普通人群中很少见,但我们的 MIS-C 患者队列富含对炎症的单基因易感性。我们的结果支持在患有 MIS-C 的既往健康儿童中使用新一代测序。



























 京公网安备 11010802027423号
京公网安备 11010802027423号