Cell Reports Physical Science ( IF 8.9 ) Pub Date : 2021-06-30 , DOI: 10.1016/j.xcrp.2021.100476 Mingming Yu , Huamin Wang , Yuhan Gao , Faxiang Bu , Hengjiang Cong , Aiwen Lei
|
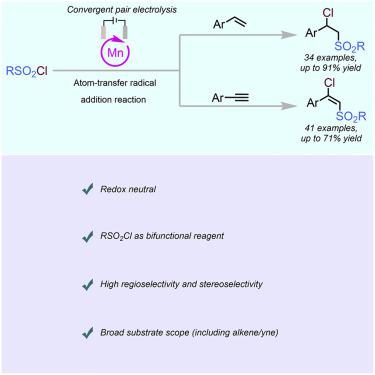
Convergent paired electrolysis is an energy-efficient model in organic synthesis, although the main obstacle with its use is matching properties of the intermediates generated in two electrodes. With the development of anodic-coupled electrosynthesis in three-component difunctionalization of alkene, a proposal for two-component difunctionalization of alkene and alkyne using a difunctional reagent has been raised. Herein, we develop a manganese-catalyzed, atom-transfer radical addition of the terminal aryl alkenes and alkynes with sulfonyl chlorides, in which manganese salt has the role of a chlorine atom-transfer catalyst as well as a redox mediator. This method generates analogs of chlorosulfonylated products of high regio- and stereoselectivity, which are derivatives of pharmaceutical drugs and natural products.
中文翻译:

通过会聚配对电解锰催化末端烯烃和炔烃的氯磺酰化
会聚配对电解是有机合成中的一种节能模型,但其使用的主要障碍是两个电极中产生的中间体的匹配特性。随着烯烃三组分双官能化阳极耦合电合成的发展,提出了使用双官能试剂对烯烃和炔烃进行双组分双官能化的建议。在此,我们开发了锰催化的末端芳基烯烃和炔烃与磺酰氯的原子转移自由基加成,其中锰盐具有氯原子转移催化剂和氧化还原介体的作用。该方法生成具有高区域选择性和立体选择性的氯磺酰化产物的类似物,它们是药物和天然产物的衍生物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号