当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Generation and Biological Evaluation of Fc Antigen Binding Fragment-Drug Conjugates as a Novel Antibody-Based Format for Targeted Drug Delivery
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2021-06-29 , DOI: 10.1021/acs.bioconjchem.1c00240 Sebastian Jäger 1, 2 , Tim R Wagner 2 , Nicolas Rasche 1 , Harald Kolmar 2 , Stefan Hecht 1 , Christian Schröter 1
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2021-06-29 , DOI: 10.1021/acs.bioconjchem.1c00240 Sebastian Jäger 1, 2 , Tim R Wagner 2 , Nicolas Rasche 1 , Harald Kolmar 2 , Stefan Hecht 1 , Christian Schröter 1
Affiliation
|
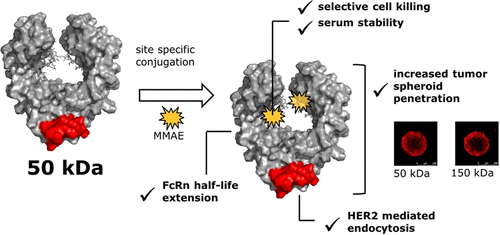
Fragment crystallizable (Fc) antigen binding fragments (Fcabs) represent a novel antibody format comprising a homodimeric Fc region with an engineered antigen binding site. In contrast to their full-length antibody offspring, Fcabs combine Fc-domain-mediated and antigen binding functions at only one-third of the size. Their reduced size is accompanied by elevated tissue penetration capabilities, which is an attractive feature for the treatment of solid tumors. In the present study, we explored for the first time Fcabs as a novel scaffold for antibody–drug conjugates (ADCs). As model, various HER2-targeting Fcab variants coupled to a pH-sensitive dye were used in internalization experiments. A selective binding on HER2-expressing tumor cells and receptor-mediated endocytosis could be confirmed for selected variants, indicating that these Fcabs meet the basic prerequisite for an ADC approach. Subsequently, Fcabs were site-specifically coupled to cytotoxic monomethyl auristatin E yielding homogeneous conjugates. The conjugates retained HER2 and FcRn binding behavior of the parent Fcabs, showed a selective in vitro cell killing and conjugation site-dependent serum stability. Moreover, Fcab conjugates showed elevated penetration in a spheroid model, compared to their full-length antibody and Trastuzumab counterparts. Altogether, the presented results emphasize the potential of Fcabs as a novel scaffold for targeted drug delivery in solid cancers and pave the way for future in vivo translation.
中文翻译:

Fc 抗原结合片段-药物偶联物的生成和生物学评价作为一种基于抗体的新型靶向药物递送形式
片段可结晶 (Fc) 抗原结合片段 (Fcabs) 代表一种新型抗体形式,包含具有工程化抗原结合位点的同型二聚体 Fc 区。与其全长抗体后代相比,Fcabs 结合了 Fc 域介导的和抗原结合功能,其大小仅为其三分之一。它们的尺寸减小伴随着组织穿透能力的提高,这是治疗实体瘤的一个有吸引力的特征。在本研究中,我们首次探索了 Fcab 作为抗体-药物偶联物 (ADC) 的新型支架。作为模型,在内化实验中使用了与 pH 敏感染料偶联的各种靶向 HER2 的 Fcab 变体。对于选定的变体,可以确认对表达 HER2 的肿瘤细胞和受体介导的内吞作用的选择性结合,表明这些 Fcab 满足 ADC 方法的基本先决条件。随后,Fcab 与细胞毒性单甲基 auristatin E 进行位点特异性偶联,产生均质的偶联物。缀合物保留了亲本 Fcab 的 HER2 和 FcRn 结合行为,显示出选择性的体外细胞杀伤和缀合位点依赖性血清稳定性。此外,与其全长抗体和曲妥珠单抗对应物相比,Fcab 偶联物在球体模型中显示出更高的渗透性。总而言之,所呈现的结果强调了 Fcabs 作为一种新型支架用于实体癌靶向药物递送的潜力,并为未来的体内转化铺平了道路。缀合物保留了亲本 Fcab 的 HER2 和 FcRn 结合行为,显示出选择性的体外细胞杀伤和缀合位点依赖性血清稳定性。此外,与其全长抗体和曲妥珠单抗对应物相比,Fcab 偶联物在球体模型中显示出更高的渗透性。总而言之,所呈现的结果强调了 Fcabs 作为一种新型支架用于实体癌靶向药物递送的潜力,并为未来的体内转化铺平了道路。缀合物保留了亲本 Fcab 的 HER2 和 FcRn 结合行为,显示出选择性的体外细胞杀伤和缀合位点依赖性血清稳定性。此外,与其全长抗体和曲妥珠单抗对应物相比,Fcab 偶联物在球体模型中显示出更高的渗透性。总而言之,所呈现的结果强调了 Fcabs 作为一种新型支架用于实体癌靶向药物递送的潜力,并为未来的体内转化铺平了道路。
更新日期:2021-08-19
中文翻译:

Fc 抗原结合片段-药物偶联物的生成和生物学评价作为一种基于抗体的新型靶向药物递送形式
片段可结晶 (Fc) 抗原结合片段 (Fcabs) 代表一种新型抗体形式,包含具有工程化抗原结合位点的同型二聚体 Fc 区。与其全长抗体后代相比,Fcabs 结合了 Fc 域介导的和抗原结合功能,其大小仅为其三分之一。它们的尺寸减小伴随着组织穿透能力的提高,这是治疗实体瘤的一个有吸引力的特征。在本研究中,我们首次探索了 Fcab 作为抗体-药物偶联物 (ADC) 的新型支架。作为模型,在内化实验中使用了与 pH 敏感染料偶联的各种靶向 HER2 的 Fcab 变体。对于选定的变体,可以确认对表达 HER2 的肿瘤细胞和受体介导的内吞作用的选择性结合,表明这些 Fcab 满足 ADC 方法的基本先决条件。随后,Fcab 与细胞毒性单甲基 auristatin E 进行位点特异性偶联,产生均质的偶联物。缀合物保留了亲本 Fcab 的 HER2 和 FcRn 结合行为,显示出选择性的体外细胞杀伤和缀合位点依赖性血清稳定性。此外,与其全长抗体和曲妥珠单抗对应物相比,Fcab 偶联物在球体模型中显示出更高的渗透性。总而言之,所呈现的结果强调了 Fcabs 作为一种新型支架用于实体癌靶向药物递送的潜力,并为未来的体内转化铺平了道路。缀合物保留了亲本 Fcab 的 HER2 和 FcRn 结合行为,显示出选择性的体外细胞杀伤和缀合位点依赖性血清稳定性。此外,与其全长抗体和曲妥珠单抗对应物相比,Fcab 偶联物在球体模型中显示出更高的渗透性。总而言之,所呈现的结果强调了 Fcabs 作为一种新型支架用于实体癌靶向药物递送的潜力,并为未来的体内转化铺平了道路。缀合物保留了亲本 Fcab 的 HER2 和 FcRn 结合行为,显示出选择性的体外细胞杀伤和缀合位点依赖性血清稳定性。此外,与其全长抗体和曲妥珠单抗对应物相比,Fcab 偶联物在球体模型中显示出更高的渗透性。总而言之,所呈现的结果强调了 Fcabs 作为一种新型支架用于实体癌靶向药物递送的潜力,并为未来的体内转化铺平了道路。


























 京公网安备 11010802027423号
京公网安备 11010802027423号