当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Measurement and Correlation of Liquid–Liquid Equilibrium Data for Ternary Systems of Water + Furfuryl Alcohol + (1-Pentanol and n-Propyl Acetate) at Different Temperatures
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-06-29 , DOI: 10.1021/acs.jced.1c00384 Yuanyuan Han 1 , Mai Han 1 , Houchun Yan 1 , Qingqing Yin 1 , Qingsong Li 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-06-29 , DOI: 10.1021/acs.jced.1c00384 Yuanyuan Han 1 , Mai Han 1 , Houchun Yan 1 , Qingqing Yin 1 , Qingsong Li 1
Affiliation
|
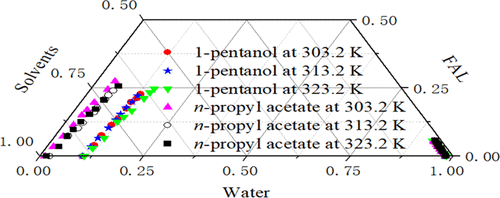
Liquid–liquid equilibrium (LLE) data for the ternary mixture of water + furfuryl alcohol (FAL) + {1-pentanol and n-propyl acetate} was measured in 101.3 kPa at 303.2, 313.2, and 323.2 K. The separation factor (S) and distribution coefficient (D) appraised the utility of extracting FAL from water. The experimental data were accurately correlated by the nonrandom two-liquid (NRTL) and the universal quasi-chemical (UNIQUAC) models with the root-mean-square deviation (RMSD%) values. The values are less than 0.37%. The relevant binary interaction parameters were calculated. The GUI-MATLAB was used to check the consistency of the calculated binary interaction parameters and liquid–liquid equilibrium data. The σ-profiles can analyze the molecular interaction, which further confirms the accuracy of the experimental results. The excellent performance of 1-pentanol and n-propyl acetate in the extraction of FAL from an aqueous solution was shown.
中文翻译:

不同温度下水+糠醇+(1-戊醇和乙酸正丙酯)三元体系的液-液平衡数据的测量和相关性
水 + 糠醇 (FAL) + {1-戊醇和乙酸正丙酯}的三元混合物的液-液平衡 (LLE) 数据在 101.3 kPa 和 303.2、313.2 和 323.2 K 下测量。分离因子 ( S ) 和分配系数 ( D) 评价了从水中提取 FAL 的效用。实验数据通过非随机二液 (NRTL) 和通用准化学 (UNIQUAC) 模型与均方根偏差 (RMSD%) 值准确关联。该值小于0.37%。计算了相关的二元相互作用参数。GUI-MATLAB用于检查计算的二元相互作用参数和液-液平衡数据的一致性。σ-profiles 可以分析分子间的相互作用,进一步证实了实验结果的准确性。显示了 1-戊醇和乙酸正丙酯在从水溶液中提取 FAL的优异性能。
更新日期:2021-07-08
中文翻译:

不同温度下水+糠醇+(1-戊醇和乙酸正丙酯)三元体系的液-液平衡数据的测量和相关性
水 + 糠醇 (FAL) + {1-戊醇和乙酸正丙酯}的三元混合物的液-液平衡 (LLE) 数据在 101.3 kPa 和 303.2、313.2 和 323.2 K 下测量。分离因子 ( S ) 和分配系数 ( D) 评价了从水中提取 FAL 的效用。实验数据通过非随机二液 (NRTL) 和通用准化学 (UNIQUAC) 模型与均方根偏差 (RMSD%) 值准确关联。该值小于0.37%。计算了相关的二元相互作用参数。GUI-MATLAB用于检查计算的二元相互作用参数和液-液平衡数据的一致性。σ-profiles 可以分析分子间的相互作用,进一步证实了实验结果的准确性。显示了 1-戊醇和乙酸正丙酯在从水溶液中提取 FAL的优异性能。



























 京公网安备 11010802027423号
京公网安备 11010802027423号