当前位置:
X-MOL 学术
›
Chem. Heterocycl. Comp.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
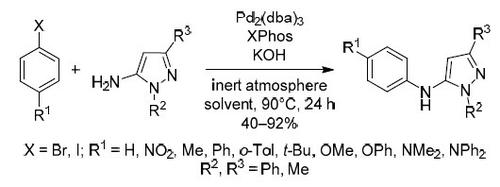
Palladium-catalyzed amino group arylation of 1,3-disubstituted 1H-pyrazol-5-amine based on Buchwald–Hartwig reaction
Chemistry of Heterocyclic Compounds ( IF 1.5 ) Pub Date : 2021-06-26 , DOI: 10.1007/s10593-021-02961-z Mateusz Kucharek , Andrzej Danel
中文翻译:

基于 Buchwald-Hartwig 反应的钯催化氨基芳基化 1,3-二取代 1H-吡唑-5-胺
更新日期:2021-06-28
Chemistry of Heterocyclic Compounds ( IF 1.5 ) Pub Date : 2021-06-26 , DOI: 10.1007/s10593-021-02961-z Mateusz Kucharek , Andrzej Danel
|
An efficient Pd-catalyzed C–N bond formation for the synthesis of different pyrazole derivatives using XPhos as a ligand and KOH as a base is presented. The developed procedure can be successfully applied for the synthesis of 5-N-aryl-1,3-disubstituted 1H-pyrazol-5-amines. Contrary to previously described procedures, this one proceeds in one step utilizing commercially available aminopyrazoles and aryl halides. Thus, a series of 5-N-aryl-1,3-disubstituted 1H-pyrazol-5-amines were obtained with satisfactory yields under optimized reaction conditions.
中文翻译:

基于 Buchwald-Hartwig 反应的钯催化氨基芳基化 1,3-二取代 1H-吡唑-5-胺
提出了一种有效的 Pd 催化的 C-N 键形成,用于使用 XPhos 作为配体和 KOH 作为碱合成不同的吡唑衍生物。所开发的程序可成功应用于5 - N-芳基-1,3-二取代1H-吡唑-5-胺的合成。与先前描述的程序相反,该程序一步利用可商购的氨基吡唑和芳基卤来进行。因此,在优化的反应条件下以令人满意的产率获得了一系列5 - N-芳基-1,3-二取代的1H-吡唑-5-胺。



























 京公网安备 11010802027423号
京公网安备 11010802027423号