Chemistry of Heterocyclic Compounds ( IF 1.5 ) Pub Date : 2021-06-26 , DOI: 10.1007/s10593-021-02967-7 Vladislav Yu. Korotaev , Savelii V. Barkovskii , Igor B. Kutyashev , Maria V. Ulitko , Alexey Yu. Barkov , Nikolay S. Zimnitskiy , Ivan А. Kochnev , Vyacheslav Ya. Sosnovskikh
|
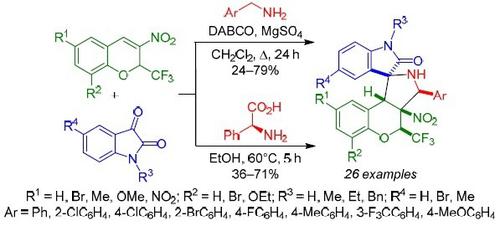
A regio- and stereoselective method for the synthesis of N-unsubstituted 3-aryl-4-(trifluoromethyl)-4H-spiro[chromeno[3,4-c]-pyrrolidine-1,3'-oxindoles] in 24–79% yields, based on the three-component reaction of 3-nitro-2-(trifluoromethyl)-2H-chromenes with azomethine ylides generated in situ from benzylamines and isatins by refluxing in CH2Cl2 for 24 h has been developed. 3-Phenyl-4-(trifluoromethyl)-4H-spiro[chromeno[3,4-c]pyrrolidine-1,3'-oxindoles] were obtained in 36–71% yields via the three-component reaction of 3-nitro-2-(trifluoromethyl)-2H-chromene, isatins, and L-phenylglycine proceeding in EtOH at 60°C for 5 h. The resulting compounds exhibited cytotoxic activity against human cervical cancer HeLa cells in the micromolar concentration range.
中文翻译:

N-未取代的 3-芳基-4-(三氟甲基)-4H-螺-[色基[3,4-c]吡咯烷-1,3'-羟吲哚]区域选择性和立体选择性合成的两种方法
在24-79 中合成N-未取代的 3-芳基-4-(三氟甲基)-4 H-螺[色基[3,4- c ]-吡咯烷-1,3'-羟吲哚]的区域选择性和立体选择性方法% 产率,基于 3-硝基-2-(三氟甲基)-2 H-色烯与由苄胺和靛红通过在 CH 2 Cl 2 中回流24 小时原位生成的偶氮甲碱叶立德的三组分反应而开发。3-Phenyl-4-(trifluoromethyl)-4 H- spiro[chromeno[3,4- c ]pyrrolidine-1,3'-oxindoles]通过3-nitro 的三组分反应以 36–71% 的产率获得-2-(三氟甲基)-2 H-色烯、靛红和 L-苯基甘氨酸在 60°C 的乙醇中处理 5 小时。所得化合物在微摩尔浓度范围内对人宫颈癌 HeLa 细胞表现出细胞毒活性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号