当前位置:
X-MOL 学术
›
Helv. Chimica Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
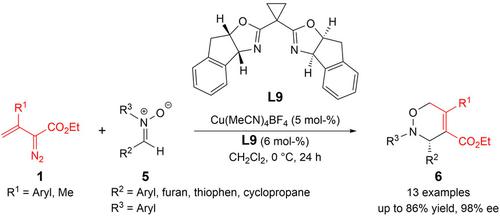
Copper(I)-Catalyzed Highly Enantioselective [3+3]-Cycloaddition of β-Aryl/Alkyl Vinyl Diazoacetates with Nitrones
Helvetica Chimica Acta ( IF 1.8 ) Pub Date : 2021-06-25 , DOI: 10.1002/hlca.202100081 Haifeng Zheng 1 , Isa Faghihi 1 , Michael P. Doyle 1
Helvetica Chimica Acta ( IF 1.8 ) Pub Date : 2021-06-25 , DOI: 10.1002/hlca.202100081 Haifeng Zheng 1 , Isa Faghihi 1 , Michael P. Doyle 1
Affiliation
|
β-Aryl/alkyl vinyl diazoacetates were investigated in metallo-vinylcarbene reactions with nitrones, revealing a Rh2(OAc)4-catalyzed cyclopropene dimerization reaction and a copper(I) catalyzed [3+3]-cycloaddition of nitrones. The chiral cyclopropyl-In-SaBox ligand with copper(I) catalysis could realize the asymmetric version of the cycloaddition reaction, delivering various 3,6-dihydro-1,2-oxazine derivatives in good yield and with excellent enantioselectivity under mild conditions.
中文翻译:

铜(I)-催化的β-芳基/烷基重氮乙酸乙烯酯与硝酮的高对映选择性[3+3]-环加成反应
在金属-乙烯基卡宾与硝酮的反应中研究了 β-芳基/烷基重氮乙酸酯,揭示了 Rh 2 (OAc) 4催化的环丙烯二聚反应和铜 (I) 催化的硝酮的 [3+3]-环加成反应。具有铜(I)催化的手性环丙基-In-SaBox配体可以实现环加成反应的不对称形式,在温和条件下以良好的收率和优异的对映选择性提供各种3,6-二氢-1,2-恶嗪衍生物。
更新日期:2021-07-16
中文翻译:

铜(I)-催化的β-芳基/烷基重氮乙酸乙烯酯与硝酮的高对映选择性[3+3]-环加成反应
在金属-乙烯基卡宾与硝酮的反应中研究了 β-芳基/烷基重氮乙酸酯,揭示了 Rh 2 (OAc) 4催化的环丙烯二聚反应和铜 (I) 催化的硝酮的 [3+3]-环加成反应。具有铜(I)催化的手性环丙基-In-SaBox配体可以实现环加成反应的不对称形式,在温和条件下以良好的收率和优异的对映选择性提供各种3,6-二氢-1,2-恶嗪衍生物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号