Chemical Engineering Journal ( IF 15.1 ) Pub Date : 2021-06-24 , DOI: 10.1016/j.cej.2021.130972 Zhenrui Wu , Jian Zou , Sadaf Shabanian , Kevin Golovin , Jian Liu
|
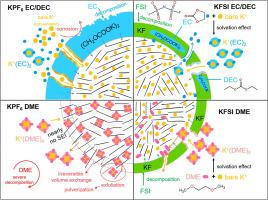
Potassium-ion battery (PIB) is a rising star in the rechargeable battery field due to its potential low cost and high energy for large-scale applications. Hard carbon (HC) is one of the most popular anodes for practical PIBs due to its high K-ion storage and relatively low material cost. However, the role of electrolytes in determining the HC performance and K-ion storage mechanism has been rarely investigated. Herein, we systematically studied the influence of four electrolyte systems, i.e., two K salts (i.e., KPF6 and KFSI) in carbonate ester and ether solvents, on the K-ion mobility, cycling stability, and charge transfer kinetics of the HC anode in PIBs. It is found that HC anode achieved the best cycling stability and kinetics performance in the KFSI EC/DEC electrolyte. Mechanismic study disclosed that the improved performance could be ascribed to the formation of robust KF-rich SEI resulting from FSI- decomposition, which effectively prevented irreversible side reactions and severe structural decay (e.g., exfoliation and pulverization). The degradation mechanisms of other electrolyte systems are also explained from the viewpoints of SEI formation and solvation/desolvation effect. It is expected that this work will provide guidance on the anode and electrolyte selection and design for PIBs in the near future.
中文翻译:

电解质化学在钾离子电池硬碳负极中的作用
钾离子电池(PIB)由于其潜在的低成本和高能量用于大规模应用而成为可充电电池领域的后起之秀。硬碳 (HC) 是实用 PIB 中最受欢迎的阳极之一,因为它具有高 K 离子存储量和相对较低的材料成本。然而,电解质在决定 HC 性能和 K 离子存储机制中的作用很少被研究。在此,我们系统地研究了四种电解质体系的影响,即两种钾盐(即KPF 6和 KFSI) 在碳酸酯和醚溶剂中,关于 PIB 中 HC 阳极的 K 离子迁移率、循环稳定性和电荷转移动力学。发现 HC 负极在 KFSI EC/DEC 电解质中实现了最佳的循环稳定性和动力学性能。Mechanismic研究公开了改进的性能可以归因于健壮KF-富SEI的从FSI所得的形成-分解,这有效地防止不可逆的副反应和严重的结构衰减(例如、剥落和粉碎)。还从SEI形成和溶剂化/去溶剂化效应的角度解释了其他电解质体系的降解机制。预计这项工作将在不久的将来为 PIB 的阳极和电解质选择和设计提供指导。


























 京公网安备 11010802027423号
京公网安备 11010802027423号