Structure ( IF 5.7 ) Pub Date : 2021-06-23 , DOI: 10.1016/j.str.2021.06.006 Hans Koss 1 , Barry Honig 2 , Lawrence Shapiro 3 , Arthur G Palmer 1
|
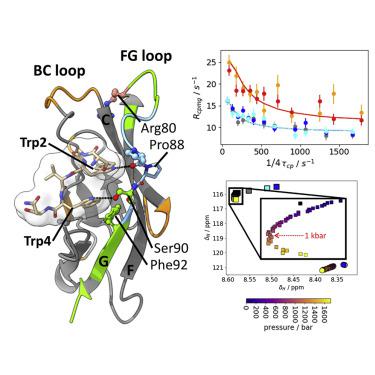
Cadherin extracellular domain 1 (EC1) mediates homophilic dimerization in adherens junctions. Conserved Trp2 and Trp4 residues in type II cadherins anchor the EC1 A strand intermolecularly in strand-swapped dimers. Herein, NMR spectroscopy is used to elucidate the roles of Trp2 and Trp4 in Cadherin-11 dimerization. The monomeric state, with the A strand and Trp side chains packed intramolecularly, is in equilibrium with sparsely populated partially and fully A-strand-exposed states, in which Trp2 (and Trp4, respectively) side-chain packing is disrupted. Exchange kinetics between the major state and the partially (fully) A-strand-exposed state is slow-intermediate (intermediate-fast). A separate very fast process exchanges ordered and random-coil BC-loop conformations with populations dependent on A-strand exposure and dimerization status. In addition, very slow processes connect the folded A-strand-exposed conformation to partially unfolded states, which may represent additional domain-swapping intermediates. The dimerization mechanism of type II cadherins is revealed as coupled folding and strand swapping.
中文翻译:

Cadherin-11的二聚化涉及多位点耦合展开和链交换
钙粘蛋白胞外结构域 1 (EC1) 介导粘附连接处的同源性二聚化。II 型钙粘蛋白中保守的 Trp2 和 Trp4 残基将 EC1 A 链锚定在链交换二聚体中。在此,NMR 光谱用于阐明 Trp2 和 Trp4 在 Cadherin-11 二聚化中的作用。A链和Trp侧链在分子内堆积的单体状态与稀疏的部分和完全A链暴露状态保持平衡,其中Trp2(和Trp4,分别)侧链堆积被破坏。主要状态和部分(完全)暴露于 A 链的状态之间的交换动力学是慢-中(中-快)。一个单独的非常快速的过程与依赖于 A 链暴露和二聚化状态的群体交换有序和随机线圈 BC 环构象。此外,非常缓慢的过程将折叠的 A 链暴露构象连接到部分未折叠状态,这可能代表额外的域交换中间体。II 型钙粘蛋白的二聚化机制被揭示为耦合折叠和链交换。



























 京公网安备 11010802027423号
京公网安备 11010802027423号