Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Deciphering Evolution Pathway of Supported NO3• Enabled via Radical Transfer from •OH to Surface NO3– Functionality for Oxidative Degradation of Aqueous Contaminants
JACS Au Pub Date : 2021-06-23 , DOI: 10.1021/jacsau.1c00124 Jongsik Kim 1 , Yun Jeong Choe 1, 2 , Sang Hoon Kim 1, 3 , In-Suk Choi 2 , Keunhong Jeong 4
JACS Au Pub Date : 2021-06-23 , DOI: 10.1021/jacsau.1c00124 Jongsik Kim 1 , Yun Jeong Choe 1, 2 , Sang Hoon Kim 1, 3 , In-Suk Choi 2 , Keunhong Jeong 4
Affiliation
|
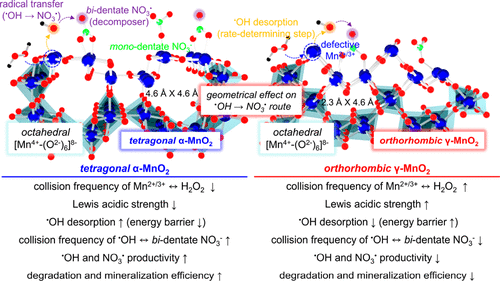
NO3• can compete with omnipotent •OH/SO4•– in decomposing aqueous pollutants because of its lengthy lifespan and significant tolerance to background scavengers present in H2O matrices, albeit with moderate oxidizing power. The generation of NO3•, however, is of grand demand due to the need of NO2•/O3, radioactive element, or NaNO3/HNO3 in the presence of highly energized electron/light. This study has pioneered a singular pathway used to radicalize surface NO3– functionalities anchored on polymorphic α-/γ-MnO2 surfaces (α-/γ-MnO2-N), in which Lewis acidic Mn2+/3+ and NO3– served to form •OH via H2O2 dissection and NO3• via radical transfer from •OH to NO3– (•OH → NO3•), respectively. The elementary steps proposed for the •OH → NO3• route could be energetically favorable and marginal except for two stages such as endothermic •OH desorption and exothermic •OH-mediated NO3– radicalization, as verified by EPR spectroscopy experiments and DFT calculations. The Lewis acidic strength of the Mn2+/3+ species innate to α-MnO2-N was the smallest among those inherent to α-/β-/γ-MnO2 and α-/γ-MnO2-N. Hence, α-MnO2-N prompted the rate-determining stage of the •OH → NO3• route (•OH desorption) in the most efficient manner, as also evidenced by the analysis on the energy barrier required to proceed with the •OH → NO3• route. Meanwhile, XANES and in situ DRIFT spectroscopy experiments corroborated that α-MnO2-N provided a larger concentration of surface NO3– species with bi-dentate binding arrays than γ-MnO2-N. Hence, α-MnO2-N could outperform γ-MnO2-N in improving the collision frequency between •OH and NO3– species and in facilitating the exothermic transition of NO3– functionalities to surface NO3• analogues per unit time. These were corroborated by a greater efficiency of α-MnO2-N in decomposing phenol, in addition to scavenging/filtration control runs and DFT calculations. Importantly, supported NO3• species provided 5–7-fold greater efficiency in degrading textile wastewater than conventional •OH and supported SO4•- analogues we discovered previously.
中文翻译:

通过从 •OH 到表面 NO3 的自由基转移来破译支持的 NO3• 的进化途径– 用于水性污染物氧化降解的功能
NO 3 •可以与万能的• OH/SO 4 •–在分解水性污染物方面竞争,因为它具有较长的使用寿命和对H 2 O 基质中存在的背景清除剂的显着耐受性,尽管具有中等氧化能力。然而,由于在高能电子/光的存在下需要 NO 2 • /O 3、放射性元素或 NaNO 3 /HNO 3,因此产生 NO 3 •的需求很大。这项研究开创了一种用于自由基化表面 NO 3的单一途径-锚定在多晶型 α-/γ-MnO 上的功能2 个表面 (α-/γ-MnO 2 -N),其中路易斯酸性 Mn 2+/3+和 NO 3 –用于形成• OH 通过 H 2 O 2分解和 NO 3 •通过自由基从• OH转移到NO 3 – ( • OH → NO 3 • ),分别。为• OH → NO 3 •路线提议的基本步骤可能在能量上是有利的和边缘的,除了两个阶段,例如吸热• OH 解吸和放热• OH 介导的 NO3 –自由基化,通过 EPR 光谱实验和 DFT 计算验证。α-MnO 2 -N固有的Mn 2+/3+物质的路易斯酸强度在α-/β-/γ-MnO 2和α-/γ-MnO 2 -N固有的那些中最小。因此,α-MnO的2 -N提示的速率决定阶段• OH→NO 3 •路线(• OH解吸)以最有效的方式,也如通过在能量势垒的分析证明与进行所需的• OH → NO 3 •路线。同时,XANES 和原位漂移光谱实验证实,与γ-MnO 2 -N相比,α-MnO 2 -N 提供了更高浓度的表面 NO 3 -具有双齿结合阵列的物质。因此,α-MnO 2 -N在提高• OH 和 NO 3 –物种之间的碰撞频率以及促进 NO 3 –官能团向表面 NO 3 •类似物的放热转变方面可以胜过 γ-MnO 2 -N 。α-MnO 2的更高效率证实了这些-N 在分解苯酚中,以及清除/过滤控制运行和 DFT 计算。重要的是,与我们之前发现的传统的• OH 和支持的 SO 4 •-类似物相比,支持的 NO 3 •物质在降解纺织废水方面的效率提高了 5-7 倍。
更新日期:2021-08-23
中文翻译:

通过从 •OH 到表面 NO3 的自由基转移来破译支持的 NO3• 的进化途径– 用于水性污染物氧化降解的功能
NO 3 •可以与万能的• OH/SO 4 •–在分解水性污染物方面竞争,因为它具有较长的使用寿命和对H 2 O 基质中存在的背景清除剂的显着耐受性,尽管具有中等氧化能力。然而,由于在高能电子/光的存在下需要 NO 2 • /O 3、放射性元素或 NaNO 3 /HNO 3,因此产生 NO 3 •的需求很大。这项研究开创了一种用于自由基化表面 NO 3的单一途径-锚定在多晶型 α-/γ-MnO 上的功能2 个表面 (α-/γ-MnO 2 -N),其中路易斯酸性 Mn 2+/3+和 NO 3 –用于形成• OH 通过 H 2 O 2分解和 NO 3 •通过自由基从• OH转移到NO 3 – ( • OH → NO 3 • ),分别。为• OH → NO 3 •路线提议的基本步骤可能在能量上是有利的和边缘的,除了两个阶段,例如吸热• OH 解吸和放热• OH 介导的 NO3 –自由基化,通过 EPR 光谱实验和 DFT 计算验证。α-MnO 2 -N固有的Mn 2+/3+物质的路易斯酸强度在α-/β-/γ-MnO 2和α-/γ-MnO 2 -N固有的那些中最小。因此,α-MnO的2 -N提示的速率决定阶段• OH→NO 3 •路线(• OH解吸)以最有效的方式,也如通过在能量势垒的分析证明与进行所需的• OH → NO 3 •路线。同时,XANES 和原位漂移光谱实验证实,与γ-MnO 2 -N相比,α-MnO 2 -N 提供了更高浓度的表面 NO 3 -具有双齿结合阵列的物质。因此,α-MnO 2 -N在提高• OH 和 NO 3 –物种之间的碰撞频率以及促进 NO 3 –官能团向表面 NO 3 •类似物的放热转变方面可以胜过 γ-MnO 2 -N 。α-MnO 2的更高效率证实了这些-N 在分解苯酚中,以及清除/过滤控制运行和 DFT 计算。重要的是,与我们之前发现的传统的• OH 和支持的 SO 4 •-类似物相比,支持的 NO 3 •物质在降解纺织废水方面的效率提高了 5-7 倍。



























 京公网安备 11010802027423号
京公网安备 11010802027423号