Solid State Sciences ( IF 3.5 ) Pub Date : 2021-06-22 , DOI: 10.1016/j.solidstatesciences.2021.106680 M. Gombotz , I. Hanghofer , S. Eisbacher-Lubensky , H.M.R. Wilkening
|
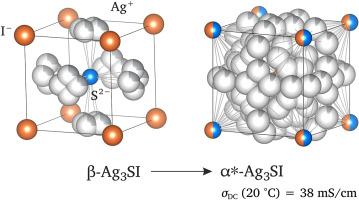
Understanding the manifold origins that lead to fast ion transport in crystalline and amorphous solids is a vital topic in materials science. To advance in this field, the study of ion dynamics in model substances, partly inspired by applications, is a suitable route to identify the most important cause of ultrafast ion exchange processes in selected classes of materials. The ternary compound Ag3SI, which crystallizes with different space groups, represents such a model substance; its complex Ag-sublattice offers many, only partly occupied and adjoining Ag+ sites that guarantee a very high Ag+ ion conductivity. Here, we used broadband impedance spectroscopy and studied the temperature stability of the ionic conductivity of the α* phase of Ag3SI at a given temperature. β-Ag3SI, synthesized by a mechanochemical approach combined with a subsequent annealing step, transforms into the α form at high temperatures. Quenching this phase leads to (metastable) α*-Ag3SI that is characterized, at 20 °C, by a conductivity σ of 38 mS cm−1. The corresponding activation energy turned out to be 200 meV. Storing α*-Ag3SI at 30 °C for 140 h causes σ to drop to ca. 15 mS cm−1 (30 °C). A more drastic decrease is seen for the sample when annealed in situ at 60 °C resulting in 9 mS cm−1 (60 °C). For comparison, the thermodynamically stable β form is characterized by 3 mS cm−1 (20 °C). This high Ag+ ion dynamics is confirmed by 109Ag NMR which yields a single, sharp line at room temperature. Importantly, we also measured electronic conductivities σeon to corroborate that σ is predominantly governed by ionic contributions. As an example, for the α* form room-temperature potentiostatic polarisation measurements yield σeon = 2.7 × 10−8 S cm−1, which is by a factor of 106 lower than its total conductivity of 38 mS cm−1. For β-Ag3SI the maximum electronic conductivity turned out to be ≈ 2.0 × 10−7 S cm−1.
中文翻译:

快速 Ag +导体α* - Ag 3 SI中的离子和电子传输
了解导致晶体和无定形固体中快速离子传输的多种起源是材料科学中的一个重要课题。为了在该领域取得进展,部分受应用启发的模型物质中离子动力学的研究是确定选定类别材料中超快离子交换过程的最重要原因的合适途径。以不同空间群结晶的三元化合物Ag 3 SI代表了这样的模型物质;其复杂的 Ag 亚晶格提供了许多仅部分占据且相邻的 Ag +位点,从而保证了非常高的 Ag +离子电导率。在这里,我们使用宽带阻抗谱研究了α离子电导率的温度稳定性。*在给定温度下的 Ag 3 SI相。β- Ag 3 SI 通过机械化学方法与后续退火步骤合成,在高温下转变为α形式。淬火该相导致(亚稳态)α * -Ag 3 SI,其特征在于在 20°C 时的电导率σ为 38 mS cm -1。相应的活化能为 200 meV。将α *-Ag 3 SI 在 30 °C 下储存140 小时会导致σ下降到大约。15 mS cm -1 (30 °C)。当原位退火时,样品的下降幅度更大在 60 °C 产生 9 mS cm -1 (60 °C)。为了比较,热力学稳定的β形式的特征为 3 mS cm -1 (20 °C)。这种高 Ag + 离子动力学由109 Ag NMR证实,其在室温下产生一条单一的、尖锐的谱线。重要的是,我们还测量了电子电导率σ eon以证实σ主要受离子贡献支配。例如,对于α * 形式的室温恒电位极化测量产生σ eon = 2.7 × 10 -8 S cm -1,其乘以 10 6低于其 38 mS cm -1 的总电导率。对于β -Ag 3 SI,最大电子电导率为≈ 2.0 × 10 -7 S cm -1。



























 京公网安备 11010802027423号
京公网安备 11010802027423号