当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Thermodynamic Studies of the Ternary System KCl–KI–H2O at 298.15 K by the Cell Potential Method
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-06-21 , DOI: 10.1021/acs.jced.1c00013 Wei Wang 1 , Shi-Hua Sang 1 , Jin-Gong Pan 2 , Gan-Hua Fu 2 , Lin Zheng 2
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-06-21 , DOI: 10.1021/acs.jced.1c00013 Wei Wang 1 , Shi-Hua Sang 1 , Jin-Gong Pan 2 , Gan-Hua Fu 2 , Lin Zheng 2
Affiliation
|
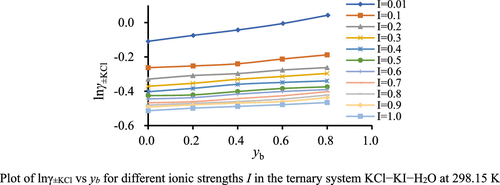
In this article, the average activity coefficients of KCl in the KCl–KI–H2O ternary system at 298.15 K were determined using the cell potential method with a cell of a potassium-ion combination ion selective electrode (ISE) and a chlorine-ion combination ion selective electrode (ISE) without a liquid junction, Also, we chose the total ionic strength I range from 0.0100 to 1.0000 mol·kg–1 for different ionic strength fractions yb of KI with yb = (0.2, 0.4, 0.6, 0.8). The experimental results show that the mean activity coefficients of KCl can be calculated well by using the Pitzer models and Nernst equation. Furthermore, the mixing Pitzer ion interaction parameters of θCl, I and ψK, I, Cl were fitted using Pitzer’s equations, and the two parameters were applied to calculated the mean activity coefficients of KI in the KCl–KI–H2O ternary system. Accordingly, the osmotic coefficients, water activities, and excess Gibbs free energies in the mixtures were studied in this mixed system.
中文翻译:

三元系统 KCl-KI-H 2 O 在 298.15 K 的热力学研究,采用细胞电位法
在本文中,KCl-KI-H 2 O 三元体系中KCl在 298.15 K的平均活度系数是使用电池电位法用钾离子组合离子选择电极 (ISE) 和氯-离子组合离子选择电极 (ISE) 不带液接界,此外,我们选择总离子强度I范围为 0.0100 至 1.0000 mol·kg –1,用于KI 的不同离子强度分数y b,y b = (0.2, 0.4, 0.6、0.8)。实验结果表明,利用Pitzer模型和Nernst方程可以很好地计算KCl的平均活度系数。此外,θ 的混合 Pitzer 离子相互作用参数Cl, I和ψ K, I, Cl使用Pitzer 方程拟合,并应用这两个参数计算KCl-KI-H 2 O 三元体系中KI 的平均活度系数。因此,在该混合体系中研究了混合物中的渗透系数、水活度和过量吉布斯自由能。
更新日期:2021-07-08
中文翻译:

三元系统 KCl-KI-H 2 O 在 298.15 K 的热力学研究,采用细胞电位法
在本文中,KCl-KI-H 2 O 三元体系中KCl在 298.15 K的平均活度系数是使用电池电位法用钾离子组合离子选择电极 (ISE) 和氯-离子组合离子选择电极 (ISE) 不带液接界,此外,我们选择总离子强度I范围为 0.0100 至 1.0000 mol·kg –1,用于KI 的不同离子强度分数y b,y b = (0.2, 0.4, 0.6、0.8)。实验结果表明,利用Pitzer模型和Nernst方程可以很好地计算KCl的平均活度系数。此外,θ 的混合 Pitzer 离子相互作用参数Cl, I和ψ K, I, Cl使用Pitzer 方程拟合,并应用这两个参数计算KCl-KI-H 2 O 三元体系中KI 的平均活度系数。因此,在该混合体系中研究了混合物中的渗透系数、水活度和过量吉布斯自由能。


























 京公网安备 11010802027423号
京公网安备 11010802027423号