当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Experimental Calorimetric Study of Thermodynamic Properties of Two Magnesium Phosphates, MgHPO4·3H2O and MgKPO4·6H2O
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-06-21 , DOI: 10.1021/acs.jced.1c00067 Maxim I. Lelet 1 , Maria L. Yakun’kova 1 , Dmitry A. Mikhailov 1 , Julia N. Lelet 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-06-21 , DOI: 10.1021/acs.jced.1c00067 Maxim I. Lelet 1 , Maria L. Yakun’kova 1 , Dmitry A. Mikhailov 1 , Julia N. Lelet 1
Affiliation
|
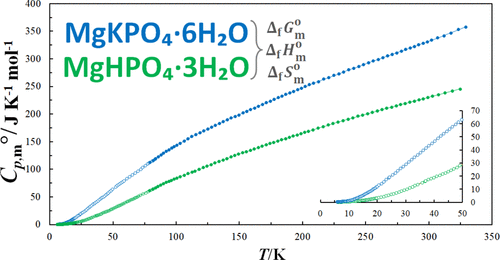
A calorimetric and thermodynamic investigation of two magnesium phosphates, MgHPO4·3H2O(cr) and MgKPO4·6H2O(cr), was undertaken. The synthesis of both phases was performed from magnesium chloride and potassium phosphate solutions at room temperature. The synthetic products were characterized by powder X-ray diffraction and X-ray fluorescence methods. The enthalpies of formation of both phases were determined using hydrofluoric acid solution calorimetry giving ΔfHmo(T = 298.15 K, MgHPO4·3H2O,cr) = −2595 ± 3 kJ mol–1 and ΔfHmo(T = 298.15 K, MgKPO4·6H2O,cr) = −3718 ± 3 kJ mol–1. The low-temperature heat capacity, Cp,mo, was measured using adiabatic calorimetry from T = 6.1 to 324.6 K for MgHPO4·3H2O(cr) and from T = 5.9 to 329.0 K for MgKPO4·6H2O(cr). Using these Cp,mo(T) data, the third law entropy at T = 298.15 K, Smo, is calculated as 212.9 ± 1.5 J K–1 mol–1 for MgHPO4·3H2O(cr) and 352.7 ± 2.1 J K–1 mol–1 for MgKPO4·6H2O(cr). These new experimental results, together with literature data, are used to calculate the Gibbs free energy of formation, ΔfGmo, for both phases, giving ΔfGmo(=298.15 K, MgHPO4·3H2O,cr) = −2292 ± 4 kJ mol–1·and ΔfGm(T = 298.15 K, MgKPO4·6H2O,cr) = −3248 ± 4 kJ mol–1. Smoothed Cp,mo(T) values between 0 and 320 K are presented, along with the values for Smo and the functions [Hmo(T) – Hmo(0)] and [Gmo(T) – Hmo(0)], for both phases.
中文翻译:

两种磷酸镁MgHPO 4 ·3H 2 O 和MgKPO 4 ·6H 2 O热力学性质的实验量热研究
对两种磷酸镁 MgHPO 4 ·3H 2 O(cr) 和 MgKPO 4 ·6H 2 O(cr) 进行了量热和热力学研究。两相的合成均在室温下由氯化镁和磷酸钾溶液进行。通过粉末X射线衍射和X射线荧光法对合成产物进行了表征。两相的形成焓使用氢氟酸溶液量热法测定,得到 Δ f H m o ( T = 298.15 K, MgHPO 4 ·3H 2 O,cr) = -2595 ± 3 kJ mol –1和 Δ f H mo ( T = 298.15 K, MgKPO 4 ·6H 2 O,cr) = -3718 ± 3 kJ mol –1。低温热容量,Ç p,米ø,使用绝热量从测定Ť = 6.1至324.6 K中MgHPO 4 ·3H 2 O(CR)和从Ť = 5.9至329.0 K中MgKPO 4 ·6H 2 ö (cr)。使用这些C p ,m o ( T ) 数据,T = 298.15 K 时的第三定律熵S m o计算为 212.9 ± 1.5 JK–1 mol –1对于 MgHPO 4 ·3H 2 O(cr) 和 352.7 ± 2.1 JK –1 mol –1对于 MgKPO 4 ·6H 2 O(cr)。这些新的实验结果与文献数据一起用于计算两相的吉布斯形成自由能 Δ f G m o,得到 Δ f G m o (=298.15 K, MgHPO 4 ·3H 2 O,cr ) = −2292 ± 4 kJ mol –1 ·和 Δ f G m ( T = 298.15 K, MgKPO 4 ·6H 2O,cr) = -3248 ± 4 kJ mol –1。显示了 0 到 320 K 之间的平滑C p ,m o ( T ) 值,以及S m o的值和函数 [ H m o ( T ) – H m o (0)] 和 [ G m o ( T ) – H m o (0)],对于两个相。
更新日期:2021-07-08
中文翻译:

两种磷酸镁MgHPO 4 ·3H 2 O 和MgKPO 4 ·6H 2 O热力学性质的实验量热研究
对两种磷酸镁 MgHPO 4 ·3H 2 O(cr) 和 MgKPO 4 ·6H 2 O(cr) 进行了量热和热力学研究。两相的合成均在室温下由氯化镁和磷酸钾溶液进行。通过粉末X射线衍射和X射线荧光法对合成产物进行了表征。两相的形成焓使用氢氟酸溶液量热法测定,得到 Δ f H m o ( T = 298.15 K, MgHPO 4 ·3H 2 O,cr) = -2595 ± 3 kJ mol –1和 Δ f H mo ( T = 298.15 K, MgKPO 4 ·6H 2 O,cr) = -3718 ± 3 kJ mol –1。低温热容量,Ç p,米ø,使用绝热量从测定Ť = 6.1至324.6 K中MgHPO 4 ·3H 2 O(CR)和从Ť = 5.9至329.0 K中MgKPO 4 ·6H 2 ö (cr)。使用这些C p ,m o ( T ) 数据,T = 298.15 K 时的第三定律熵S m o计算为 212.9 ± 1.5 JK–1 mol –1对于 MgHPO 4 ·3H 2 O(cr) 和 352.7 ± 2.1 JK –1 mol –1对于 MgKPO 4 ·6H 2 O(cr)。这些新的实验结果与文献数据一起用于计算两相的吉布斯形成自由能 Δ f G m o,得到 Δ f G m o (=298.15 K, MgHPO 4 ·3H 2 O,cr ) = −2292 ± 4 kJ mol –1 ·和 Δ f G m ( T = 298.15 K, MgKPO 4 ·6H 2O,cr) = -3248 ± 4 kJ mol –1。显示了 0 到 320 K 之间的平滑C p ,m o ( T ) 值,以及S m o的值和函数 [ H m o ( T ) – H m o (0)] 和 [ G m o ( T ) – H m o (0)],对于两个相。



























 京公网安备 11010802027423号
京公网安备 11010802027423号