Chemosphere ( IF 8.8 ) Pub Date : 2021-06-22 , DOI: 10.1016/j.chemosphere.2021.131306 Qiang Sun 1 , Xuebin Hu 2 , Huaili Zheng 1 , Yanyan An 1 , Jinyao Qu 2 , Zhanmei Zhang 3 , Sarfaraz Khan 1
|
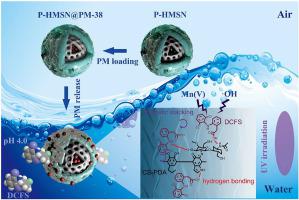
In this work, the novel hollow mesoporous coagulant was prepared by chitosan-polydopamine coating and permanganate loading into silica nanoparticles for investigating the simultaneous enrichment and degradation of diclofenac sodium (DCFS) combined with ultraviolet irradiation. The enrichment kinetic of DCFS was explained well with pseudo-second-order model, indicating the exist of hydrogen bonding. Based on the correlation coefficients, the enriched isotherms were fitted by models which accorded with the BET > Freundlich > Langmuir sequence. The result showed that, in addition to the coagulant and DCFS, there were aromatic stackings among DCFS molecules. Due to both effects of which, the DCFS enrichment could be realized significantly in the range of pH 4.0–9.0. It was degraded at the copresence of ultraviolet and permanganate released from coagulant in acidic aqueous medium. The release mechanism was simulated through Korsmayer-Peppas model, implying case-II transport and Fickian diffusion. Additionally, Mn (V) and •OH radicals were vital in the DCFS degradation process. The coagulant could be reloaded at least ten times and that from each cycle was used directly for DCFS removal for six times without rinse process, which provided a potential application in environmental remediation.
中文翻译:

二氧化硅基中空介孔混凝剂释放高锰酸盐结合紫外线对双氯芬酸钠的时空富集和降解
在这项工作中,通过壳聚糖-聚多巴胺涂层和高锰酸盐负载到二氧化硅纳米颗粒中制备了新型中空介孔混凝剂,以研究双氯芬酸钠 (DCFS) 结合紫外线照射的同时富集和降解。DCFS的富集动力学用伪二级模型很好地解释,表明存在氢键。根据相关系数,通过符合BET>Freundlich>Langmuir序列的模型拟合富集等温线。结果表明,除混凝剂和DCFS外,DCFS分子之间还存在芳香堆积。由于这两种影响,在pH 4.0-9.0范围内可以显着实现DCFS富集。在酸性水介质中混凝剂释放出的紫外线和高锰酸盐共同作用下降解。释放机制是通过 Korsmayer-Peppas 模型模拟的,这意味着 case-II 传输和 Fickian 扩散。此外,Mn (V) 和 •OH 自由基在 DCFS 降解过程中至关重要。混凝剂至少可以重新加载10次,并且每个循环的混凝剂直接用于DCFS去除6次,无需冲洗过程,这为环境修复提供了潜在的应用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号