Progress in Neurobiology ( IF 6.7 ) Pub Date : 2021-06-21 , DOI: 10.1016/j.pneurobio.2021.102110 Seung Jae Hyeon 1 , Jinyoung Park 2 , Junsang Yoo 3 , Su-Hyun Kim 3 , Yu Jin Hwang 3 , Seung-Chan Kim 4 , Tian Liu 5 , Hyun Soo Shim 3 , Yunha Kim 3 , Yakdol Cho 6 , Jiwan Woo 6 , Key-Sun Kim 7 , Richard H Myers 8 , Hannah L Ryu 9 , Neil W Kowall 10 , Eun Joo Song 11 , Eun Mi Hwang 4 , Hyemyung Seo 12 , Junghee Lee 10 , Hoon Ryu 13
|
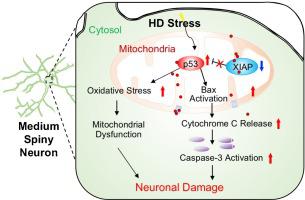
Mitochondrial dysfunction is associated with neuronal damage in Huntington’s disease (HD), but the precise mechanism of mitochondria-dependent pathogenesis is not understood yet. Herein, we found that colocalization of XIAP and p53 was prominent in the cytosolic compartments of normal subjects but reduced in HD patients and HD transgenic animal models. Overexpression of mutant Huntingtin (mHTT) reduced XIAP levels and elevated mitochondrial localization of p53 in striatal cells in vitro and in vivo. Interestingly, XIAP interacted directly with the C-terminal domain of p53 and decreased its stability via autophagy. Overexpression of XIAP prevented mitochondrially targeted-p53 (Mito-p53)-induced mitochondrial oxidative stress and striatal cell death, whereas, knockdown of XIAP exacerbated Mito-p53-induced neuronal damage in vitro. In vivo transduction of AAV-shRNA XIAP in the dorsal striatum induced rapid onset of disease and reduced the lifespan of HD transgenic (N171-82Q) mice compared to WT littermate mice. XIAP dysfunction led to ultrastructural changes of the mitochondrial cristae and nucleus morphology in striatal cells. Knockdown of XIAP exacerbated neuropathology and motor dysfunctions in N171-82Q mice. In contrast, XIAP overexpression improved neuropathology and motor behaviors in both AAV-mHTT-transduced mice and N171-82Q mice. Our data provides a molecular and pathological mechanism that deregulation of XIAP triggers mitochondria dysfunction and other neuropathological processes via the neurotoxic effect of p53 in HD. Together, the XIAP-p53 pathway is a novel pathological marker and can be a therapeutic target for improving the symptoms in HD.
中文翻译:

X连锁凋亡蛋白抑制剂(XIAP)的功能障碍通过改变亨廷顿病中的p53活性触发神经病理学过程
线粒体功能障碍与亨廷顿病 (HD) 的神经元损伤有关,但线粒体依赖性发病机制的确切机制尚不清楚。在这里,我们发现 XIAP 和 p53 的共定位在正常受试者的细胞溶质区室中很突出,但在 HD 患者和 HD 转基因动物模型中减少。在体外和体内,突变体亨廷顿蛋白 (mHTT) 的过表达降低了 XIAP 水平并提高了 p53 在纹状体细胞中的线粒体定位。有趣的是,XIAP 直接与 p53 的 C 端结构域相互作用并通过降低其稳定性自噬。XIAP 的过表达阻止了线粒体靶向 p53 (Mito-p53) 诱导的线粒体氧化应激和纹状体细胞死亡,而 XIAP 的敲低在体外加剧了 Mito-p53 诱导的神经元损伤。体内与 WT 同窝小鼠相比,在背侧纹状体中转导 AAV-shRNA XIAP 可诱导疾病的快速发作并缩短 HD 转基因 (N171-82Q) 小鼠的寿命。XIAP功能障碍导致纹状体细胞线粒体嵴和细胞核形态的超微结构变化。XIAP 的敲除加剧了 N171-82Q 小鼠的神经病理学和运动功能障碍。相比之下,XIAP 过表达改善了 AAV-mHTT 转导小鼠和 N171-82Q 小鼠的神经病理学和运动行为。我们的数据提供了一种分子和病理机制,即 XIAP 的失调通过以下方式触发线粒体功能障碍和其他神经病理学过程p53 在 HD 中的神经毒性作用。总之,XIAP-p53 通路是一种新的病理标志物,可以作为改善 HD 症状的治疗靶点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号