当前位置:
X-MOL 学术
›
Int. J. Numer. Method. Biomed. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Computational model of silica nanoparticle penetration into tumor spheroids: Effects of methoxy and carboxy PEG surface functionalization and hyperthermia
International Journal for Numerical Methods in Biomedical Engineering ( IF 2.1 ) Pub Date : 2021-06-20 , DOI: 10.1002/cnm.3504 Abhignyan Nagesetti 1 , George S Dulikravich 2 , Helcio R B Orlande 3 , Marcelo J Colaco 3 , Anthony J McGoron 1
International Journal for Numerical Methods in Biomedical Engineering ( IF 2.1 ) Pub Date : 2021-06-20 , DOI: 10.1002/cnm.3504 Abhignyan Nagesetti 1 , George S Dulikravich 2 , Helcio R B Orlande 3 , Marcelo J Colaco 3 , Anthony J McGoron 1
Affiliation
|
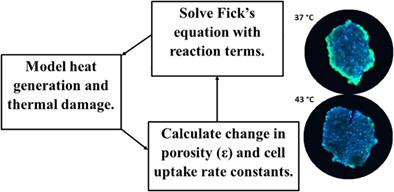
Drug delivery to tumors suffers from poor solubility, specificity, diffusion through the tumor micro-environment and nonoptimal interactions with components of the extracellular matrix and cell surface receptors. Nanoparticles and drug–polymer complexes address many of these problems. However, large size exasperates the problem of slow diffusion through the tumor. Three-dimensional tumor spheroids are good models to evaluate approaches to mitigate these difficulties and aid in design strategies to improve the delivery of drugs to treat cancer effectively. Diffusion of drug carriers is highly dependent on cell uptake rate parameters (association/dissociation) and temperature. Hyperthermia increases molecular transport and is known to act synergistically with chemotherapy to improve treatment. This study presents a new inverse estimation approach based on Bayesian probability for estimating nanoparticle cell uptake rates from experiments. The parameters were combined with a finite element computational model of nanoparticle transport under hyperthermia conditions to explore its effect on tumor porosity, diffusion and particle binding (association and dissociation) at cell surfaces. Carboxy-PEG-silane (cPEGSi) nanoparticles showed higher cell uptake compared to methoxy-PEG-silane (mPEGSi) nanoparticles. Simulations were consistent with experimental results from Skov-3 ovarian cancer spheroids. Amorphous silica (cPEGSi) nanoparticles (58 nm) concentrated at the periphery of the tumor spheroids at 37°C but mild hyperthermia (43°C) increased nanoparticle penetration. Thus, hyperthermia may enhance cancer treatment by improving blood delivery to tumors, enhancing extravasation and penetration into tumors, trigger release of drug from the carrier at the tumor site and possibly lead to synergistic anti-cancer activity with the drug.
中文翻译:

二氧化硅纳米粒子渗透到肿瘤球体的计算模型:甲氧基和羧基 PEG 表面功能化和热疗的影响
向肿瘤递送药物的缺点是溶解性、特异性、通过肿瘤微环境的扩散以及与细胞外基质和细胞表面受体成分的非最佳相互作用。纳米粒子和药物-聚合物复合物解决了许多这些问题。然而,大尺寸加剧了通过肿瘤扩散缓慢的问题。三维肿瘤球体是评估减轻这些困难的方法的良好模型,并有助于设计策略以改善有效治疗癌症的药物递送。药物载体的扩散高度依赖于细胞摄取速率参数(结合/解离)和温度。热疗增加了分子转运,并且已知与化疗协同作用以改善治疗。本研究提出了一种基于贝叶斯概率的新逆估计方法,用于从实验中估计纳米颗粒细胞摄取率。这些参数与高温条件下纳米颗粒传输的有限元计算模型相结合,以探索其对细胞表面肿瘤孔隙率、扩散和颗粒结合(结合和解离)的影响。与甲氧基-PEG-硅烷(mPEGSi)纳米颗粒相比,羧基-PEG-硅烷(cPEGSi)纳米颗粒显示出更高的细胞摄取。模拟结果与 Skov-3 卵巢癌球体的实验结果一致。无定形二氧化硅 (cPEGSi) 纳米粒子 (58 nm) 在 37°C 下集中在肿瘤球体的外围,但温和的热疗 (43°C) 增加了纳米粒子的渗透。因此,
更新日期:2021-08-10
中文翻译:

二氧化硅纳米粒子渗透到肿瘤球体的计算模型:甲氧基和羧基 PEG 表面功能化和热疗的影响
向肿瘤递送药物的缺点是溶解性、特异性、通过肿瘤微环境的扩散以及与细胞外基质和细胞表面受体成分的非最佳相互作用。纳米粒子和药物-聚合物复合物解决了许多这些问题。然而,大尺寸加剧了通过肿瘤扩散缓慢的问题。三维肿瘤球体是评估减轻这些困难的方法的良好模型,并有助于设计策略以改善有效治疗癌症的药物递送。药物载体的扩散高度依赖于细胞摄取速率参数(结合/解离)和温度。热疗增加了分子转运,并且已知与化疗协同作用以改善治疗。本研究提出了一种基于贝叶斯概率的新逆估计方法,用于从实验中估计纳米颗粒细胞摄取率。这些参数与高温条件下纳米颗粒传输的有限元计算模型相结合,以探索其对细胞表面肿瘤孔隙率、扩散和颗粒结合(结合和解离)的影响。与甲氧基-PEG-硅烷(mPEGSi)纳米颗粒相比,羧基-PEG-硅烷(cPEGSi)纳米颗粒显示出更高的细胞摄取。模拟结果与 Skov-3 卵巢癌球体的实验结果一致。无定形二氧化硅 (cPEGSi) 纳米粒子 (58 nm) 在 37°C 下集中在肿瘤球体的外围,但温和的热疗 (43°C) 增加了纳米粒子的渗透。因此,

























 京公网安备 11010802027423号
京公网安备 11010802027423号