当前位置:
X-MOL 学术
›
Part. Part. Syst. Charact.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
In Situ Self-Assembly of Quantum Dots at the Plasma Membrane Mediates Energy Transfer-Based Activation of Channelrhodopsin
Particle & Particle Systems Characterization ( IF 2.7 ) Pub Date : 2021-06-19 , DOI: 10.1002/ppsc.202100053 Okhil K. Nag 1 , Megan E. Muroski 1 , Lauren D. Field 1, 2 , Michael H. Stewart 3 , Eunkeu Oh 3 , Kimihiro Susumu 3, 4 , Joseph R. Spangler 2 , Scott A. Walper 1 , James B. Delehanty 1
Particle & Particle Systems Characterization ( IF 2.7 ) Pub Date : 2021-06-19 , DOI: 10.1002/ppsc.202100053 Okhil K. Nag 1 , Megan E. Muroski 1 , Lauren D. Field 1, 2 , Michael H. Stewart 3 , Eunkeu Oh 3 , Kimihiro Susumu 3, 4 , Joseph R. Spangler 2 , Scott A. Walper 1 , James B. Delehanty 1
Affiliation
|
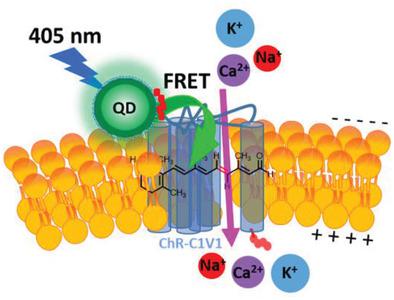
The use of nanoparticle (NP) bioconjugates to control the activity of membrane ion channels has recently emerged as a new paradigm for the activation of electrically excitable cells. An NP-based strategy is reported for the specific activation of channelrhodopsin C1V1 (ChR-C1V1) expressed in the plasma membrane of HEK 293T/17 cells. Hydrophilic CdSe/ZnS core–shell semiconductor quantum dots (QDs) are self-assembled to the exofacial face of recombinantly expressed ChR-C1V1 by metal affinity-driven interaction of the QD ZnS shell with an N-terminal hexahistidine tag displayed on ChR-C1V1. This configuration enables the Förster resonance energy transfer (FRET)-based excitation and activation of the 11-cis-retinal moiety of ChR-C1V1 using the QD as a light harvesting transducer/energy donor. It is shown that the specific laser-induced opening of the ChR-C1V1 channel wherein the photoexcited QD (405 nm excitation, 530 nm emission) iteratively activates ChR-C1V1 channels as confirmed using the voltage-sensitive dye (VSD) bis-(1,3-diethylthiobarbituric acid)trimethine oxonol (DiSBAC2(3)). In the absence of the QD transducer, excitation of ChR-C1V1-expressing cells at 405 nm results in no activation of ChR-C1V1. The results demonstrate the ability to controllably interface QDs with living cells for the activation of ChR membrane proteins and detail a new NP-bioconjugate hybrid system for the specific activation of ion channels.
中文翻译:

质膜上量子点的原位自组装介导基于能量转移的视紫红质激活
使用纳米颗粒 (NP) 生物结合物来控制膜离子通道的活性,最近已成为激活电可兴奋细胞的新范例。据报道,一种基于 NP 的策略用于特异性激活 HEK 293T/17 细胞质膜中表达的视紫红质 C1V1 (ChR-C1V1)。亲水性 CdSe/ZnS 核壳半导体量子点 (QD) 通过 QD ZnS 壳与ChR-C1V1 上显示的N端六组氨酸标签的金属亲和驱动相互作用自组装到重组表达的 ChR- C1V1 的外表面. 这种配置能够基于 Förster 共振能量转移 (FRET) 激发和激活 11-顺式-ChR-C1V1 的视网膜部分,使用 QD 作为光收集传感器/能量供体。结果表明,特定的激光诱导 ChR-C1V1 通道打开,其中光激发的 QD(405 nm 激发,530 nm 发射)反复激活 ChR-C1V1 通道,如使用电压敏感染料 (VSD) bis-(1 ,3-二乙基硫代巴比妥酸)trimethine oxonol (DiSBAC 2 (3))。在没有 QD 传感器的情况下,在 405 nm 处激发表达 ChR-C1V1 的细胞不会导致 ChR-C1V1 的激活。结果证明了将量子点与活细胞可控连接以激活 ChR 膜蛋白的能力,并详细说明了用于特定激活离子通道的新 NP-生物共轭混合系统。
更新日期:2021-07-19
中文翻译:

质膜上量子点的原位自组装介导基于能量转移的视紫红质激活
使用纳米颗粒 (NP) 生物结合物来控制膜离子通道的活性,最近已成为激活电可兴奋细胞的新范例。据报道,一种基于 NP 的策略用于特异性激活 HEK 293T/17 细胞质膜中表达的视紫红质 C1V1 (ChR-C1V1)。亲水性 CdSe/ZnS 核壳半导体量子点 (QD) 通过 QD ZnS 壳与ChR-C1V1 上显示的N端六组氨酸标签的金属亲和驱动相互作用自组装到重组表达的 ChR- C1V1 的外表面. 这种配置能够基于 Förster 共振能量转移 (FRET) 激发和激活 11-顺式-ChR-C1V1 的视网膜部分,使用 QD 作为光收集传感器/能量供体。结果表明,特定的激光诱导 ChR-C1V1 通道打开,其中光激发的 QD(405 nm 激发,530 nm 发射)反复激活 ChR-C1V1 通道,如使用电压敏感染料 (VSD) bis-(1 ,3-二乙基硫代巴比妥酸)trimethine oxonol (DiSBAC 2 (3))。在没有 QD 传感器的情况下,在 405 nm 处激发表达 ChR-C1V1 的细胞不会导致 ChR-C1V1 的激活。结果证明了将量子点与活细胞可控连接以激活 ChR 膜蛋白的能力,并详细说明了用于特定激活离子通道的新 NP-生物共轭混合系统。


























 京公网安备 11010802027423号
京公网安备 11010802027423号