Immunity, Inflammation and Disease ( IF 2.493 ) Pub Date : 2021-06-19 , DOI: 10.1002/iid3.428 Cataldo Patruno 1 , Maddalena Napolitano 2 , Luca Stingeni 3 , Gabriella Fabbrocini 4
|
Recently, Food and Drug Administration and European Medicines Agency approved the BNT162b2 messenger RNA (mRNA) vaccine for the prevention of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (coronavirus disease 2019 [COVID-19]). The vaccine is administered in two doses separated by 21 days.1 The Centres for Disease Control and Prevention identified the onset of anaphylaxis in 21 out of 1,893,360 subjects receiving the first vaccine dose.2 Furthermore, pruritic rash and/or mild respiratory symptoms were observed in 83 of them.2 All these adverse reactions mostly appeared within the first 30 min after the vaccination and can likely be interpreted as immunoglobulin E-mediated hypersensitivity, hypothetically related to the vaccine component polyethylene glycol 2000.2, 3 To date, no delayed cutaneous adverse events have been reported, other than injection site inflammation.
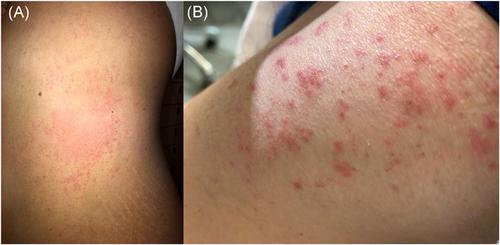
Herein, we described two patients with skin rash appearing several days after the first doses of the vaccine. A 42-year-old woman with no history of allergies or skin diseases developed acute urticaria (AU) on the trunk and limbs (Figure 1A). Wheals started 7 days after vaccine administration. Systemic antihistamine treatment alone was inefficacious, while the addition of prednisone (25 mg/day) for 1 week led to healing. The second patient was a 55-year-old healthy man with multiple itchy erythematous papules, vesicles, and blisters on the buttocks and extensor surface of the extremities (Figure 1B). The rash appeared about 10 days after vaccination. Skin histology showed spongiosis, epidermal exocytosis of lymphocytes, and apoptotic keratinocytes, besides dermal edema. These clinical and histologic features were consistent with an erythema multiforme (EM)-like eruption. The eruption disappeared after 10 days of treatment with systemic prednisone (25 mg/day). Both the patients denied infections or intake of drugs after the vaccine administration, thus leading to the suspicion of association between exposure to the vaccine and the appearance of the rashes. Delayed maculopapular reactions have been described after the administration of conventional vaccines for bacterial or viral diseases.4 These reactions may be due to both the antimicrobial or additional components of the drug.4 However, there are no reports regarding delayed urticarial or EM-like eruptions.4 On the other hand, AU and EM-like eruption may occur in some patients affected with COVID-19.5 At least some of these cases are considered related to viral infection and not to concomitant treatments.5

BNT162b2 is a nucleoside-modified mRNA vaccine that induces transient expression of the SARS-CoV-2 spike antigen (SA) and subsequent neutralizing antibody production and cellular response against the virus.1 It could be assumed that, in our two patients, the expression of SA induced by the BNT162b2 vaccine might have induced both AU and EM-like eruptions. Therefore, the pathogenesis might be similar to that occurring in COVID-19 patients developing these rashes. Obviously, it is essential to collect data regarding a large population to evaluate the effective association of delayed urticarial or EM-like eruptions with the BNT162b2 vaccine. Furthermore, such cases might also be useful in evaluating the immune response and efficacy of vaccination for SARS-CoV-2.
中文翻译:

SARS-CoV-2 疫苗接种后出现皮疹:哪种关系(如果有)?
最近,美国食品药品监督管理局和欧洲药品管理局批准了 BNT162b2 信使 RNA(mRNA)疫苗,用于预防严重急性呼吸综合征冠状病毒 2(SARS-CoV-2)感染(冠状病毒病 2019 [COVID-19])。该疫苗分两次接种,间隔 21 天。1疾病控制和预防中心确定在接受第一剂疫苗的 1,893,360 名受试者中有 21 名发生过敏反应。2此外,在其中 83 人中观察到瘙痒性皮疹和/或轻度呼吸道症状。2所有这些不良反应大多出现在疫苗接种后的前 30 分钟内,可能被解释为免疫球蛋白 E 介导的超敏反应,假设与疫苗成分聚乙二醇 2000 相关。2, 3迄今为止,尚未报告任何延迟性皮肤不良事件,注射部位炎症除外。
在此,我们描述了两名在第一次接种疫苗后几天出现皮疹的患者。一名 42 岁女性,没有过敏史或皮肤病史,躯干和四肢出现急性荨麻疹 (AU)(图 1A)。接种疫苗后 7 天开始出现风团。单独全身性抗组胺药治疗无效,而加入泼尼松(25 毫克/天)1 周后可治愈。第二位患者是一名 55 岁的健康男性,臀部和四肢伸肌表面有多个发痒的红斑丘疹、水疱和水疱(图 1B)。接种疫苗后约 10 天出现皮疹。皮肤组织学显示,除真皮水肿外,还有海绵状组织增生、淋巴细胞表皮胞吐和角质形成细胞凋亡。这些临床和组织学特征与多形性红斑 (EM) 样皮疹一致。用全身泼尼松(25 毫克/天)治疗 10 天后,皮疹消失。两名患者在接种疫苗后均否认感染或服用过药物,因此怀疑接种疫苗与出现皮疹之间存在关联。已经描述了在接种细菌或病毒疾病的常规疫苗后出现延迟性黄斑丘疹反应。4这些反应可能是由于药物的抗菌成分或其他成分造成的。4然而,没有关于延迟性荨麻疹或 EM 样出疹的报告。4另一方面,一些感染 COVID-19 的患者可能会出现 AU 和 EM 样喷发。5这些病例中至少有一些被认为与病毒感染有关,而与伴随治疗无关。5

BNT162b2 是一种核苷修饰的 mRNA 疫苗,可诱导 SARS-CoV-2 刺突抗原 (SA) 的瞬时表达,随后中和抗体的产生和针对病毒的细胞反应。1可以假设,在我们的两名患者中,BNT162b2 疫苗诱导的 SA 表达可能诱导了 AU 和 EM 样喷发。因此,发病机制可能与出现这些皮疹的 COVID-19 患者的发病机制相似。显然,必须收集大量人群的数据以评估延迟性荨麻疹或 EM 样皮疹与 BNT162b2 疫苗的有效关联。此外,此类病例也可能有助于评估 SARS-CoV-2 疫苗接种的免疫反应和功效。


























 京公网安备 11010802027423号
京公网安备 11010802027423号