Biochimica et Biophysica Acta (BBA) - Molecular Cell Research ( IF 5.1 ) Pub Date : 2021-06-19 , DOI: 10.1016/j.bbamcr.2021.119082 Roberto Campagna 1 , Łukasz Mateuszuk 2 , Kamila Wojnar-Lason 3 , Patrycja Kaczara 2 , Anna Tworzydło 2 , Agnieszka Kij 2 , Robert Bujok 4 , Jacek Mlynarski 4 , Yu Wang 5 , Davide Sartini 6 , Monica Emanuelli 6 , Stefan Chlopicki 3
|
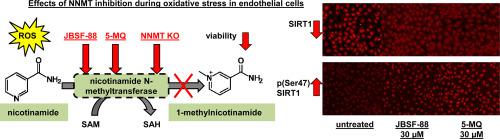
Nicotinamide N-methyltransferase (NNMT, EC 2.1.1.1.) plays an important role in the growth of many different tumours and is also involved in various non-neoplastic disorders. However, the presence and role of NNMT in the endothelium has yet to be specifically explored. Here, we characterized the functional activity of NNMT in the endothelium and tested whether NNMT regulates endothelial cell viability. NNMT in endothelial cells (HAEC, HMEC-1 and EA.hy926) was inhibited using two approaches: pharmacological inhibition of the enzyme by NNMT inhibitors (5-amino-1-methylquinoline – 5MQ and 6-methoxynicotinamide – JBSF-88) or by shRNA-mediated silencing. Functional inhibition of NNMT was confirmed by LC/MS/MS-based analysis of impaired MNA production. The effects of NNMT inhibition on cellular viability were analyzed in both the absence and presence of menadione.
Our results revealed that all studied endothelial lines express relatively high levels of functionally active NNMT compared with cancer cells (MDA-MB-231). Although the aldehyde oxidase 1 enzyme was also expressed in the endothelium, the further metabolites of N1-methylnicotinamide (N1-methyl-2-pyridone-5-carboxamide and N1-methyl-4-pyridone-3-carboxamide) generated by this enzyme were not detected, suggesting that endothelial NNMT-derived MNA was not subsequently metabolized in the endothelium by aldehyde oxidase 1. Menadione induced a concentration-dependent decrease in endothelial viability as evidenced by a decrease in cell number that was associated with the upregulation of NNMT and SIRT1 expression in the nucleus in viable cells. The suppression of the NNMT activity either by NNMT inhibitors or shRNA-based silencing significantly decreased the endothelial cell viability in response to menadione. Furthermore, NNMT inhibition resulted in nuclear SIRT1 expression downregulation and upregulation of the phosphorylated form of SIRT1 on Ser47. In conclusion, our results suggest that the endothelial nuclear NNMT/SIRT1 pathway exerts a cytoprotective role that safeguards endothelial cell viability under oxidant stress insult.
中文翻译:

内皮中的烟酰胺 N-甲基转移酶可防止氧化应激引起的内皮损伤
烟酰胺N-甲基转移酶(NNMT,EC 2.1.1.1.)在许多不同肿瘤的生长中起重要作用,并且还涉及各种非肿瘤性疾病。然而,NNMT 在内皮中的存在和作用还有待专门研究。在这里,我们表征了内皮中 NNMT 的功能活性,并测试了 NNMT 是否调节内皮细胞活力。使用两种方法抑制内皮细胞(HAEC、HMEC-1 和 EA.hy926)中的 NNMT:通过 NNMT 抑制剂(5-氨基-1-甲基喹啉 - 5MQ 和 6-甲氧基烟酰胺 - JBSF-88)或通过shRNA 介导的沉默。NNMT 的功能抑制通过基于 LC/MS/MS 的 MNA 生成受损分析得到证实。在甲萘醌不存在和存在的情况下分析了 NNMT 抑制对细胞活力的影响。
我们的结果表明,与癌细胞 (MDA-MB-231) 相比,所有研究的内皮细胞系都表达相对高水平的功能活性 NNMT。尽管醛氧化酶 1 酶也在内皮中表达,但由该酶产生的 N1-甲基烟酰胺(N1-methyl-2-pyridone-5-carboxamide 和 N1-methyl-4-pyridone-3-carboxamide)的进一步代谢物是未检测到,表明内皮 NNMT 衍生的 MNA 随后不会在内皮中被醛氧化酶 1 代谢。甲萘醌诱导内皮活力的浓度依赖性降低,这可以通过与 NNMT 和 SIRT1 上调相关的细胞数量减少来证明在活细胞的细胞核中表达。NNMT 抑制剂或基于 shRNA 的沉默对 NNMT 活性的抑制显着降低了响应甲萘醌的内皮细胞活力。此外,NNMT 抑制导致核 SIRT1 表达下调和 Ser47 上 SIRT1 磷酸化形式的上调。总之,我们的结果表明内皮细胞核 NNMT/SIRT1 通路发挥细胞保护作用,在氧化应激损伤下保护内皮细胞活力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号