当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Comparing pathways for electricity-based production of dimethoxymethane as a sustainable fuel
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2021-6-18 , DOI: 10.1039/d1ee00689d Jannik Burre 1, 2, 3, 4 , Dominik Bongartz 1, 2, 3, 4 , Sarah Deutz 2, 4, 5, 6 , Chalachew Mebrahtu 2, 3, 4, 7 , Ole Osterthun 2, 3, 4, 7 , Ruiyan Sun 2, 3, 4, 7 , Simon Völker 2, 4, 5, 6 , André Bardow 2, 4, 5, 6, 8 , Jürgen Klankermayer 2, 3, 4, 7 , Regina Palkovits 2, 3, 4, 7 , Alexander Mitsos 1, 2, 3, 4, 8
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2021-6-18 , DOI: 10.1039/d1ee00689d Jannik Burre 1, 2, 3, 4 , Dominik Bongartz 1, 2, 3, 4 , Sarah Deutz 2, 4, 5, 6 , Chalachew Mebrahtu 2, 3, 4, 7 , Ole Osterthun 2, 3, 4, 7 , Ruiyan Sun 2, 3, 4, 7 , Simon Völker 2, 4, 5, 6 , André Bardow 2, 4, 5, 6, 8 , Jürgen Klankermayer 2, 3, 4, 7 , Regina Palkovits 2, 3, 4, 7 , Alexander Mitsos 1, 2, 3, 4, 8
Affiliation
|
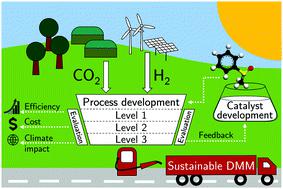
Synthetic dimethoxymethane (DMM) is a promising fuel or blend component as it offers outstanding combustion characteristics. DMM production from hydrogen (H2) and carbon dioxide (CO2) is technically feasible with established technology but results in a low overall process efficiency. Recent research in catalyst development has increased DMM yield significantly and new reaction pathways have been proposed. Yet, it remains unknown how the achievements in catalyst development affect process performance. To close this gap, we analyze processes based on five reaction pathways regarding exergy efficiency, production cost, and climate impact. As the pathways have different technology readiness levels, we develop a methodology that ensures consistent boundary conditions and model detail between pathways. The methodology enables a hierarchical optimization-based process design and evaluation. The results show that the non-oxidative (i.e., reductive, dehydrogenative, and transfer-hydrogenative) pathways consume stoichiometrically less H2 not only than the established and oxidative pathway, but also less than most other electricity-based fuels (e-fuels). The higher resource efficiency of these pathways increases process exergy efficiency from 75% to 84%; production cost (2.1$ Ldiesel-eq.−1) becomes competitive to other e-fuels; and the impact on climate change reduces by up to 92% compared to fossil diesel, if renewable electricity is utilized. Whereas the reductive pathway may already enable a sustainable production of DMM with only little catalyst improvements, the dehydrogenative and transfer-hydrogenative pathways still require a higher DMM selectivity and methanol conversion, respectively. With considerable catalyst improvements, a maximum exergy efficiency of 92% and minimum production cost of 2.0$ Ldiesel-eq.−1 are achievable. Our analyses show: With the non-oxidative pathways, the high potential of DMM is no longer restricted to its outstanding combustion characteristics but extended to its production.
中文翻译:

比较基于电力生产二甲氧基甲烷作为可持续燃料的途径
合成二甲氧基甲烷 (DMM) 是一种很有前途的燃料或混合成分,因为它具有出色的燃烧特性。从氢气 (H 2 ) 和二氧化碳 (CO 2 )生产 DMM) 使用现有技术在技术上是可行的,但会导致整体流程效率低下。最近在催化剂开发方面的研究显着提高了 DMM 的产率,并提出了新的反应途径。然而,催化剂开发的成就如何影响工艺性能仍然未知。为了弥补这一差距,我们根据有关火用效率、生产成本和气候影响的五种反应途径分析了过程。由于路径具有不同的技术准备水平,我们开发了一种方法来确保路径之间的边界条件和模型细节一致。该方法支持基于层次优化的过程设计和评估。结果表明,非氧化性(即、还原、脱氢和转移加氢)途径消耗的 H 2化学计量比既定和氧化途径少,而且比大多数其他基于电力的燃料(电子燃料)消耗的 H 2少。这些途径的更高资源效率将过程火用效率从 75% 提高到 84%;生产成本(2.1$ L柴油当量-1) 与其他电子燃料相比具有竞争力;如果使用可再生电力,与化石柴油相比,对气候变化的影响最多可减少 92%。虽然还原途径可能已经能够在催化剂改进很小的情况下实现 DMM 的可持续生产,但脱氢和转移氢化途径仍然分别需要更高的 DMM 选择性和甲醇转化率。随着催化剂的显着改进,92% 的最大火用效率和 2.0 美元 L柴油当量的最低生产成本。-1是可以实现的。我们的分析表明:通过非氧化途径,DMM 的高潜力不再局限于其出色的燃烧特性,而是扩展到其生产。
更新日期:2021-06-18
中文翻译:

比较基于电力生产二甲氧基甲烷作为可持续燃料的途径
合成二甲氧基甲烷 (DMM) 是一种很有前途的燃料或混合成分,因为它具有出色的燃烧特性。从氢气 (H 2 ) 和二氧化碳 (CO 2 )生产 DMM) 使用现有技术在技术上是可行的,但会导致整体流程效率低下。最近在催化剂开发方面的研究显着提高了 DMM 的产率,并提出了新的反应途径。然而,催化剂开发的成就如何影响工艺性能仍然未知。为了弥补这一差距,我们根据有关火用效率、生产成本和气候影响的五种反应途径分析了过程。由于路径具有不同的技术准备水平,我们开发了一种方法来确保路径之间的边界条件和模型细节一致。该方法支持基于层次优化的过程设计和评估。结果表明,非氧化性(即、还原、脱氢和转移加氢)途径消耗的 H 2化学计量比既定和氧化途径少,而且比大多数其他基于电力的燃料(电子燃料)消耗的 H 2少。这些途径的更高资源效率将过程火用效率从 75% 提高到 84%;生产成本(2.1$ L柴油当量-1) 与其他电子燃料相比具有竞争力;如果使用可再生电力,与化石柴油相比,对气候变化的影响最多可减少 92%。虽然还原途径可能已经能够在催化剂改进很小的情况下实现 DMM 的可持续生产,但脱氢和转移氢化途径仍然分别需要更高的 DMM 选择性和甲醇转化率。随着催化剂的显着改进,92% 的最大火用效率和 2.0 美元 L柴油当量的最低生产成本。-1是可以实现的。我们的分析表明:通过非氧化途径,DMM 的高潜力不再局限于其出色的燃烧特性,而是扩展到其生产。



























 京公网安备 11010802027423号
京公网安备 11010802027423号