Current Nanoscience ( IF 1.5 ) Pub Date : 2021-05-01 , DOI: 10.2174/1573413716999200820144001 Xin Zheng 1 , Xinyue Zhang 1 , Qili Hu 2 , Hongbin Sun 2 , Linshan Wang 2 , Xiaowu Li 1
|
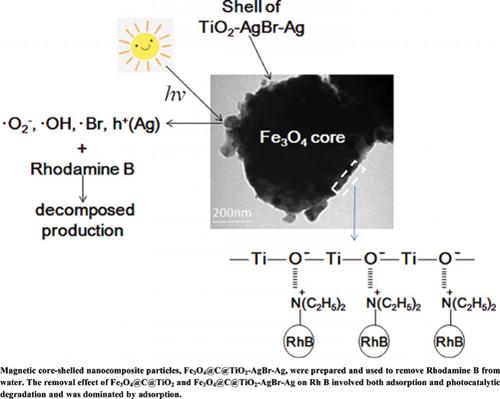
Background: TiO2 nanoparticles possess adsorption capacity and photocatalytic activity, and are thus fitted for removal of dyes from water. However, TiO2 nanoparticles are difficult to separate from the bulk solution due to high loss. Moreover, TiO2 can only use light with a wavelength of less than 387.5 nm, so the utilization efficiency of solar energy is very low. The present work prepared Fe3O4@C@TiO2-AgBr-Ag composites to overcome the shortcomings of TiO2.
Objective: Adsorptive and photocatalytic performance of nano-magnetic materials Fe3O4@C@TiO2- AgBr-Ag.
Methods: Fe3O4@C@TiO2 and Fe3O4@C@TiO2-AgBr-Ag magnetic nanocomposites were prepared by the sol-gel method. Their structure was characterized. Performances of Fe3O4@C@TiO2 and Fe3O4@C@TiO2-AgBr-Ag for removing Rh B were thoroughly investigated and compared. Langmuir– Hinshelwood kinetic model was applied to analyze the heterogeneous processes of adsorption and photodegradation.
Results: Removal experiments were carried out with Rhodamine B as the subject. The effects of contacting time, pH, subject concentration, and doses of photocatalyst on the removal performance were studied. The removal of Rh B by Fe3O4@C@TiO2 and Fe3O4@C@TiO2-AgBr-Ag involved both adsorption and photodegradation, and the photocatalytic activity of Fe3O4@C@TiO2-AgBr-Ag was much higher than that of Fe3O4@C@TiO2. The optimum removal conditions were determined. Under the optimal conditions, the removal rate of Rhodamine B with Fe3O4@C@TiO2 was 77.8%, and the removal rate of Rhodamine B with Fe3O4@C@TiO2- AgBr-Ag was 87.3%.
Conclusion: The coupling of the nanostructured metal Ag to the outer surface of TiO2 could effectively increase photocatalytic efficiency under visible light. The photocatalysts could be separated from bulk solutions by using a magnet and be easily recycled. The removal reaction kinetics fitted with the first-order model.
中文翻译:

纳米磁性材料Fe 3 O 4 @C@TiO 2 -AgBr-Ag对罗丹明B的吸附及光催化活性
背景:TiO 2纳米粒子具有吸附能力和光催化活性,因此适合从水中去除染料。然而,由于高损失,TiO 2纳米颗粒难以从本体溶液中分离。而且,TiO 2只能利用波长小于387.5nm的光,所以太阳能的利用效率很低。目前的工作制备了 Fe 3 O 4 @C@TiO 2 -AgBr-Ag 复合材料,以克服 TiO 2的缺点。
目的:纳米磁性材料Fe 3 O 4 @C@TiO 2 - AgBr-Ag的吸附和光催化性能。
方法:采用溶胶-凝胶法制备Fe 3 O 4 @C@TiO 2和Fe 3 O 4 @C@TiO 2 -AgBr-Ag磁性纳米复合材料。它们的结构被表征。对Fe 3 O 4 @C@TiO 2和Fe 3 O 4 @C@TiO 2 -AgBr-Ag去除Rh B的性能进行了深入研究和比较。Langmuir-Hinshelwood 动力学模型用于分析吸附和光降解的非均相过程。
结果:以罗丹明B为对象进行去除实验。研究了接触时间、pH、对象浓度和光催化剂剂量对去除性能的影响。Fe 3 O 4 @C@TiO 2和Fe 3 O 4 @C@TiO 2 -AgBr-Ag对Rh B的去除涉及吸附和光降解,以及Fe 3 O 4 @C@TiO 2 -的光催化活性AgBr-Ag 远高于 Fe 3 O 4 @C@TiO 2。确定了最佳去除条件。在最佳条件下,罗丹明B对Fe的去除率3 O 4 @C@TiO 2为77.8%,罗丹明B与Fe 3 O 4 @C@TiO 2 - AgBr-Ag的去除率为87.3%。
结论:纳米结构金属Ag与TiO 2外表面的偶联可有效提高可见光下的光催化效率。光催化剂可以通过使用磁铁从本体溶液中分离出来,并且很容易回收利用。去除反应动力学符合一级模型。



























 京公网安备 11010802027423号
京公网安备 11010802027423号