当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Fly Ash–Ca(OH)2 Reactivity in Hypersaline NaCl and CaCl2 Brines
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2021-06-17 , DOI: 10.1021/acssuschemeng.1c01884 Marie Collin 1, 2 , Dale P. Prentice 1, 2 , Ross A. Arnold 1, 2 , Kirk Ellison 3 , Dante A. Simonetti 2, 4 , Gaurav N. Sant 1, 2, 5, 6
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2021-06-17 , DOI: 10.1021/acssuschemeng.1c01884 Marie Collin 1, 2 , Dale P. Prentice 1, 2 , Ross A. Arnold 1, 2 , Kirk Ellison 3 , Dante A. Simonetti 2, 4 , Gaurav N. Sant 1, 2, 5, 6
Affiliation
|
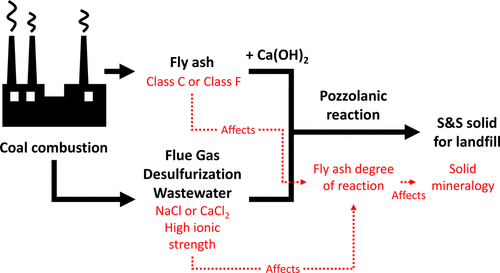
The disposal of highly concentrated brines from coal power generation can be effectively accomplished by physical solidification and chemical stabilization (S&S) processes that utilize fly ashes as a reactant. Herein, pozzolanic fly ashes are typically combined with calcium-based additives to achieve S&S. While the reactions of fly ash–(cement)–water systems have been extensively studied, the reactivity of fly ashes in hypersaline brines (ionic strength, Im > 1 mol/L) is comparatively less understood. Therefore, the interactions of a Class C (Ca-rich) fly ash and a Class F (Ca-poor) fly ash were examined in the presence of Ca(OH)2, and their thermodynamic phase equilibria were modeled on contact with NaCl or CaCl2 brines for 0 ≤ Im ≤ 7.5 mol/L. At low ionic strengths (<0.3 mol/L), reactivity and stable phase assemblages remain effectively unaltered. However, at high(er) ionic strengths (>0.5 mol/L), the phase assemblage shows a particular abundance of Cl-AFm compounds (i.e., Kuzel’s and Friedel’s salts). Although formation of Kuzel’s and Friedel’s salts enhances the Class F fly ash reactions in both NaCl and CaCl2 brines, NaCl brines compromise Class C fly ash reactivity substantially, while CaCl2 results in the reactivity remaining essentially unchanged. Thermodynamic modeling that accounts for the fractional and noncongruent dissolution of the fly ashes indicates that their differences in reaction behavior are provoked by differences in the prevalent pore solution pH, which affects phase stability. The outcomes offer new insights for matching fly ashes, Ca additives, and brines, and accounting for and controlling fly ash–brine interactions as relevant to optimizing physical solidification and chemical stabilization applications.
中文翻译:

飞灰-Ca(OH) 2在高盐 NaCl 和 CaCl 2盐水中的反应性
使用飞灰作为反应物的物理固化和化学稳定 (S&S) 工艺可以有效地处理燃煤发电产生的高浓度卤水。在此,火山灰飞灰通常与钙基添加剂结合以实现 S&S。虽然飞灰-(水泥)-水系统的反应已被广泛研究,但对飞灰在高盐盐水中的反应性(离子强度,I m > 1 mol/L)的了解相对较少。因此,在 Ca(OH) 2存在下检查了 C 类(富含钙)飞灰和 F 类(贫钙)飞灰的相互作用,并且它们的热力学相平衡模拟了与 NaCl 或CaCl 2盐水 0 ≤ I米≤7.5摩尔/ L。在低离子强度 (<0.3 mol/L) 下,反应性和稳定相组合保持有效不变。然而,在高(er)离子强度(>0.5 mol/L)下,相组合显示出特别丰富的 Cl-AFm 化合物(即 Kuzel 和 Friedel 盐)。尽管 Kuzel 和 Friedel 盐的形成增强了 NaCl 和 CaCl 2盐水中的F 类飞灰反应,但NaCl 盐水显着损害了 C 类飞灰的反应性,而 CaCl 2导致反应性基本保持不变。考虑飞灰的部分和非一致溶解的热力学模型表明,它们反应行为的差异是由影响相稳定性的普遍孔隙溶液 pH 值的差异引起的。结果为匹配飞灰、钙添加剂和盐水提供了新的见解,并解释和控制与优化物理固化和化学稳定应用相关的飞灰-盐水相互作用。
更新日期:2021-06-28
中文翻译:

飞灰-Ca(OH) 2在高盐 NaCl 和 CaCl 2盐水中的反应性
使用飞灰作为反应物的物理固化和化学稳定 (S&S) 工艺可以有效地处理燃煤发电产生的高浓度卤水。在此,火山灰飞灰通常与钙基添加剂结合以实现 S&S。虽然飞灰-(水泥)-水系统的反应已被广泛研究,但对飞灰在高盐盐水中的反应性(离子强度,I m > 1 mol/L)的了解相对较少。因此,在 Ca(OH) 2存在下检查了 C 类(富含钙)飞灰和 F 类(贫钙)飞灰的相互作用,并且它们的热力学相平衡模拟了与 NaCl 或CaCl 2盐水 0 ≤ I米≤7.5摩尔/ L。在低离子强度 (<0.3 mol/L) 下,反应性和稳定相组合保持有效不变。然而,在高(er)离子强度(>0.5 mol/L)下,相组合显示出特别丰富的 Cl-AFm 化合物(即 Kuzel 和 Friedel 盐)。尽管 Kuzel 和 Friedel 盐的形成增强了 NaCl 和 CaCl 2盐水中的F 类飞灰反应,但NaCl 盐水显着损害了 C 类飞灰的反应性,而 CaCl 2导致反应性基本保持不变。考虑飞灰的部分和非一致溶解的热力学模型表明,它们反应行为的差异是由影响相稳定性的普遍孔隙溶液 pH 值的差异引起的。结果为匹配飞灰、钙添加剂和盐水提供了新的见解,并解释和控制与优化物理固化和化学稳定应用相关的飞灰-盐水相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号