当前位置:
X-MOL 学术
›
Environ. Pollut.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanism of As(III) removal properties of biochar-supported molybdenum-disulfide/iron-oxide system
Environmental Pollution ( IF 8.9 ) Pub Date : 2021-06-16 , DOI: 10.1016/j.envpol.2021.117600 Zulqarnain Haider Khan 1 , Minling Gao 1 , Jingjie Wu 1 , Ran Bi 2 , Ch Tahir Mehmood 3 , Zhengguo Song 1
Environmental Pollution ( IF 8.9 ) Pub Date : 2021-06-16 , DOI: 10.1016/j.envpol.2021.117600 Zulqarnain Haider Khan 1 , Minling Gao 1 , Jingjie Wu 1 , Ran Bi 2 , Ch Tahir Mehmood 3 , Zhengguo Song 1
Affiliation
|
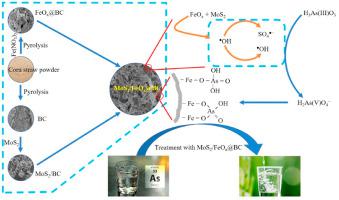
Sulfate (SO) and hydroxyl-based (HO) radical are considered potential agents for As(III) removal from aquatic environments. We have reported the synergistic role of SO and HO radicals for As(III) removal via facile synthesis of biochar-supported SO species. MoS−modified biochar (MoS/BC), iron oxide-biochar (FeO@BC), and MoS−modified iron oxide-biochar (MoS/FeO@BC) were prepared and systematically characterized to understand the underlying mechanism for arsenic removal. The MoS/FeOx@BC displayed much higher As(III) adsorption (27 mg/g) compared to MoS/BC (7 mg/g) and FeOx@BC (12 mg/g). Effects of kinetics, As(III) concentration, temperature, and pH were also investigated. The adsorption of As(III) by MoS/FeOx@BC followed the Freundlich adsorption isotherm and pseudo-second-order, indicating multilayer adsorption and chemisorption, respectively. The FTIR and XPS analysis confirmed the presence of Fe–O bonds and SO groups in the MoS/FeO@BC. Electron paramagnetic resonance (EPR) and radical quenching experiments have shown the generation of SO radicals as predominant species in the presence of MoS and FeO in MoS/FeOx@BC via radical transfer from HO to SO. The HO and SO radicals synergistically contributed to enhanced As(III) removal. It is envisaged that As(III) initially adsorbed through electrostatic interactions and partially undergoes oxidation, which is finally adsorbed to MoS/FeOx@BC after being oxidized to As(V). The MoS/FeO@BC system could be considered a novel material for effective removal of As(III) from aqueous environments owing to its cost-effective synthesis and easy scalability for actual applications.
中文翻译:

生物炭负载二硫化钼/氧化铁体系的As(III)去除性能机理
硫酸盐 (SO) 和羟基 (H2O) 自由基被认为是从水生环境中去除 As(III) 的潜在试剂。我们报道了 SO 和 H2O 自由基通过轻松合成生物炭支持的 SO 物种去除 As(III) 的协同作用。制备了 MoS2 改性生物炭 (MoS2/BC)、氧化铁生物炭 (FeO@BC) 和 MoS2 改性氧化铁生物炭 (MoS2/FeO@BC) 并进行系统表征,以了解除砷的基本机制。与 MoS2/BC (7 mg/g) 和 FeOx@BC (12 mg/g) 相比,MoS2/FeOx@BC 显示出更高的 As(III) 吸附量 (27 mg/g)。还研究了动力学、As(III) 浓度、温度和 pH 值的影响。 MoS2/FeOx@BC对As(III)的吸附遵循Freundlich吸附等温线和准二级吸附,分别表明多层吸附和化学吸附。 FTIR 和 XPS 分析证实 MoS2/FeO@BC 中存在 Fe-O 键和 SO 基团。电子顺磁共振(EPR)和自由基猝灭实验表明,在 MoS2/FeOx@BC 中存在 MoS 和 FeO 的情况下,通过从 H2O 到 SO 的自由基转移,生成了 SO 自由基作为主要物种。 H2O 和 SO 自由基协同促进 As(III) 去除。预计As(III)最初通过静电相互作用被吸附,并部分发生氧化,最终被氧化为As(V)后被MoS/FeOx@BC吸附。 MoS2/FeO@BC系统可以被认为是一种从水环境中有效去除As(III)的新型材料,因为其合成成本效益高且易于在实际应用中扩展。
更新日期:2021-06-16
中文翻译:

生物炭负载二硫化钼/氧化铁体系的As(III)去除性能机理
硫酸盐 (SO) 和羟基 (H2O) 自由基被认为是从水生环境中去除 As(III) 的潜在试剂。我们报道了 SO 和 H2O 自由基通过轻松合成生物炭支持的 SO 物种去除 As(III) 的协同作用。制备了 MoS2 改性生物炭 (MoS2/BC)、氧化铁生物炭 (FeO@BC) 和 MoS2 改性氧化铁生物炭 (MoS2/FeO@BC) 并进行系统表征,以了解除砷的基本机制。与 MoS2/BC (7 mg/g) 和 FeOx@BC (12 mg/g) 相比,MoS2/FeOx@BC 显示出更高的 As(III) 吸附量 (27 mg/g)。还研究了动力学、As(III) 浓度、温度和 pH 值的影响。 MoS2/FeOx@BC对As(III)的吸附遵循Freundlich吸附等温线和准二级吸附,分别表明多层吸附和化学吸附。 FTIR 和 XPS 分析证实 MoS2/FeO@BC 中存在 Fe-O 键和 SO 基团。电子顺磁共振(EPR)和自由基猝灭实验表明,在 MoS2/FeOx@BC 中存在 MoS 和 FeO 的情况下,通过从 H2O 到 SO 的自由基转移,生成了 SO 自由基作为主要物种。 H2O 和 SO 自由基协同促进 As(III) 去除。预计As(III)最初通过静电相互作用被吸附,并部分发生氧化,最终被氧化为As(V)后被MoS/FeOx@BC吸附。 MoS2/FeO@BC系统可以被认为是一种从水环境中有效去除As(III)的新型材料,因为其合成成本效益高且易于在实际应用中扩展。


























 京公网安备 11010802027423号
京公网安备 11010802027423号