当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kinetics of Decomposition of PFOS Relevant to Thermal Desorption Remediation of Soils
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2021-06-16 , DOI: 10.1021/acs.iecr.1c01504 Nathan H. Weber 1 , Sebastian P. Stockenhuber 1 , Cameron S. Delva 1 , Ammar Abu Fara 1 , Charles C. Grimison 2 , John A. Lucas 1 , John C. Mackie 1 , Michael Stockenhuber 1 , Eric M. Kennedy 1
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2021-06-16 , DOI: 10.1021/acs.iecr.1c01504 Nathan H. Weber 1 , Sebastian P. Stockenhuber 1 , Cameron S. Delva 1 , Ammar Abu Fara 1 , Charles C. Grimison 2 , John A. Lucas 1 , John C. Mackie 1 , Michael Stockenhuber 1 , Eric M. Kennedy 1
Affiliation
|
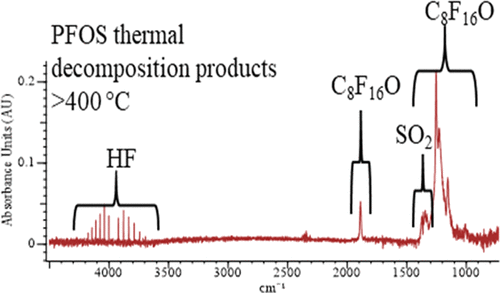
Kinetics of pyrolysis of the pollutant perfluorooctanesulfonic acid (PFOS) in inert bath gases have been studied in two flow reactors constructed of α-alumina and of stainless steel (SS) at temperatures between 400 and 615 °C. Results from the SS reactor give support to previous and our own quantum chemical calculations based on smaller perfluorinated sulfonates, according to which initiation of decomposition of PFOS first takes place by elimination of HF to form an unstable α-sultone with a rate constant, k1. The sultone then rapidly liberates SO2 and forms perfluorooctanoyl fluoride with a rate constant, k2 with k2 ≫ k1 such that the overall rate constant k′ ≈ k1. Products observed from both reactors in the above temperature range comprised HF, SO2, and perfluorooctanoyl fluoride. The value of the rate constant for the formation of HF and SO2 measured in the SS reactor was found to be k1 = (1.3 ± 0.5) × 1014 exp(−253 ± 5 kJ/mol/RT) s–1.
中文翻译:

与土壤热解吸修复相关的全氟辛烷磺酸分解动力学
在由 α-氧化铝和不锈钢 (SS) 构成的两个流动反应器中,在 400 到 615 °C 的温度下研究了污染物全氟辛烷磺酸 (PFOS) 在惰性浴气中的热解动力学。SS 反应器的结果支持先前和我们自己基于较小全氟磺酸盐的量子化学计算,根据该计算,PFOS 的分解首先发生在消除 HF 以形成速率常数k 1的不稳定 α-磺内酯. 然后磺内酯迅速释放 SO 2并形成速率常数为k 2 的全氟辛酰氟,其中k 2 ≫ k 1使得总速率常数k ′ ≈ k 1。在上述温度范围内从两个反应器观察到的产物包括HF、SO 2和全氟辛酰氟。发现在 SS 反应器中测量的 HF 和 SO 2形成的速率常数值为k 1 = (1.3 ± 0.5) × 10 14 exp(-253 ± 5 kJ/mol/ RT ) s –1。
更新日期:2021-06-30
中文翻译:

与土壤热解吸修复相关的全氟辛烷磺酸分解动力学
在由 α-氧化铝和不锈钢 (SS) 构成的两个流动反应器中,在 400 到 615 °C 的温度下研究了污染物全氟辛烷磺酸 (PFOS) 在惰性浴气中的热解动力学。SS 反应器的结果支持先前和我们自己基于较小全氟磺酸盐的量子化学计算,根据该计算,PFOS 的分解首先发生在消除 HF 以形成速率常数k 1的不稳定 α-磺内酯. 然后磺内酯迅速释放 SO 2并形成速率常数为k 2 的全氟辛酰氟,其中k 2 ≫ k 1使得总速率常数k ′ ≈ k 1。在上述温度范围内从两个反应器观察到的产物包括HF、SO 2和全氟辛酰氟。发现在 SS 反应器中测量的 HF 和 SO 2形成的速率常数值为k 1 = (1.3 ± 0.5) × 10 14 exp(-253 ± 5 kJ/mol/ RT ) s –1。


























 京公网安备 11010802027423号
京公网安备 11010802027423号