当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photocatalytic Reduction and Crystallization Hybrid System for Removal and Recovery of Lead (Pb)
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2021-06-14 , DOI: 10.1021/acs.iecr.1c01169 Vivek Kumar 1 , Ravinder K. Wanchoo 1 , Amrit P. Toor 1
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2021-06-14 , DOI: 10.1021/acs.iecr.1c01169 Vivek Kumar 1 , Ravinder K. Wanchoo 1 , Amrit P. Toor 1
Affiliation
|
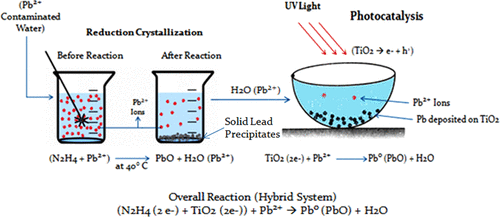
The heavy metal lead (Pb) is toxic to humans, animals, and plants and shows persistence and is nondegradable in aquatic media. A novel method has been proposed using photocatalysis (PC) and reduction crystallization (RC) for efficient removal and recovery of lead (Pb) from the aqueous phase. Using photocatalysis, maximum removal of lead ions (Pb2+) was 79.6% under optimized conditions (2 g L–1 TiO2, pH 5.3, and 35 W cm–2 UV light intensity). However, reduction crystallization removed 90% lead ions (Pb2+) using hydrazine hydrate as a reduction agent with optimized parameters (pH 10 and temp 80 °C). To achieve maximum removal as well as recovery of toxic lead, both methods, photocatalysis and reduction crystallization, were combined, removing 98.2% of lead ions (Pb2+) from the aqueous phase. The recovered precipitates and photocatalysts (TiO2) were analyzed using scanning electron microscopy–energy-dispersive X-ray spectroscopy (SEM-EDS), X-ray diffraction (XRD), and Fourier transform infrared (FT-IR) techniques after applying processes at optimized conditions. The EDS spectrum showed that the recovered precipitates and TiO2 contained the lead (Pb) element. The XRD peak analysis showed that precipitates contained lead having the crystallite size of around 90 nm, whereas the XRD spectrum of recovered titanium dioxide indicated additional peaks at specific angles, showing lead deposition on the TiO2 surface. The rate of photocatalytic removal of lead ions (Pb2+) followed the Langmuir–Hinshelwood equation of the first order.
中文翻译:

用于去除和回收铅 (Pb) 的光催化还原和结晶混合系统
重金属铅 (Pb) 对人类、动物和植物有毒,具有持久性,在水生介质中不可降解。已经提出了一种使用光催化 (PC) 和还原结晶 (RC) 从水相中有效去除和回收铅 (Pb) 的新方法。使用光催化,在优化条件(2 g L –1 TiO 2,pH 5.3 和 35 W cm –2紫外光强度)下,铅离子 (Pb 2+ ) 的最大去除率为 79.6% 。然而,还原结晶去除了 90% 的铅离子(Pb 2+) 使用水合肼作为具有优化参数(pH 10 和温度 80 °C)的还原剂。为了最大限度地去除和回收有毒铅,将光催化和还原结晶这两种方法相结合,从水相中去除了 98.2% 的铅离子 (Pb 2+ )。使用扫描电子显微镜-能量色散 X 射线光谱 (SEM-EDS)、X 射线衍射 (XRD) 和傅里叶变换红外 (FT-IR) 技术分析回收的沉淀物和光催化剂 (TiO 2 )。在优化条件下。EDS 谱表明回收的沉淀物和 TiO 2含有铅 (Pb) 元素。XRD 峰分析表明,沉淀物含有晶粒尺寸约为 90 nm 的铅,而回收的二氧化钛的 XRD 谱表明在特定角度有额外的峰,表明铅沉积在 TiO 2表面。光催化去除铅离子 (Pb 2+ )的速率遵循一阶 Langmuir-Hinshelwood 方程。
更新日期:2021-06-23
中文翻译:

用于去除和回收铅 (Pb) 的光催化还原和结晶混合系统
重金属铅 (Pb) 对人类、动物和植物有毒,具有持久性,在水生介质中不可降解。已经提出了一种使用光催化 (PC) 和还原结晶 (RC) 从水相中有效去除和回收铅 (Pb) 的新方法。使用光催化,在优化条件(2 g L –1 TiO 2,pH 5.3 和 35 W cm –2紫外光强度)下,铅离子 (Pb 2+ ) 的最大去除率为 79.6% 。然而,还原结晶去除了 90% 的铅离子(Pb 2+) 使用水合肼作为具有优化参数(pH 10 和温度 80 °C)的还原剂。为了最大限度地去除和回收有毒铅,将光催化和还原结晶这两种方法相结合,从水相中去除了 98.2% 的铅离子 (Pb 2+ )。使用扫描电子显微镜-能量色散 X 射线光谱 (SEM-EDS)、X 射线衍射 (XRD) 和傅里叶变换红外 (FT-IR) 技术分析回收的沉淀物和光催化剂 (TiO 2 )。在优化条件下。EDS 谱表明回收的沉淀物和 TiO 2含有铅 (Pb) 元素。XRD 峰分析表明,沉淀物含有晶粒尺寸约为 90 nm 的铅,而回收的二氧化钛的 XRD 谱表明在特定角度有额外的峰,表明铅沉积在 TiO 2表面。光催化去除铅离子 (Pb 2+ )的速率遵循一阶 Langmuir-Hinshelwood 方程。


























 京公网安备 11010802027423号
京公网安备 11010802027423号