当前位置:
X-MOL 学术
›
Chemistryopen
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reaction of Tertiary 2-Chloroketones with Cyanide Ions: Application to 3-Chloroquinolinediones
ChemistryOpen ( IF 2.3 ) Pub Date : 2021-06-14 , DOI: 10.1002/open.202100024 Antonín Klásek 1 , Stanislav Kafka 1 , Ondřej Rudolf 1 , Antonín Lyčka 2 , Michal Rouchal 1 , Lukáš Bednář 1
ChemistryOpen ( IF 2.3 ) Pub Date : 2021-06-14 , DOI: 10.1002/open.202100024 Antonín Klásek 1 , Stanislav Kafka 1 , Ondřej Rudolf 1 , Antonín Lyčka 2 , Michal Rouchal 1 , Lukáš Bednář 1
Affiliation
|
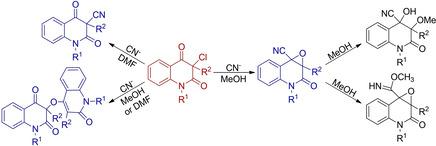
3-Chloroquinoline-2,4-diones react with cyanide ions in dimethyl formamide to give 3-cyanoquinoline-2,4-diones in small yields due to the strong hindrance of the substituent at the C-3 atom. Good yields can be achieved if the substituent at this position is the methyl group. In the methanol solution, the reaction proceeds by an addition mechanism to form 2-oxo-1a,2,3,7b-tetrahydrooxireno[2,3-c]quinoline-7b-carbonitriles, from which 4-hydroxy-3-methoxy-2-oxo-1,2,3,4-tetrahydroquinoline-4-carbonitriles are subsequently formed by opening of the epoxide ring with methanol. Some minor products of these reactions have also been isolated. The 1H, 13C and 15N NMR spectra of the prepared compounds were measured, and all resonances were assigned using appropriate two-dimensional spectra.
中文翻译:

叔 2-氯酮与氰离子的反应:应用于 3-氯喹啉二酮
由于 C-3 原子上取代基的强位阻,3-Chloroquinoline-2,4-diones 与二甲基甲酰胺中的氰离子反应生成 3-cyanoquinoline-2,4-diones,产率很小。如果该位置的取代基是甲基,则可以获得良好的收率。在甲醇溶液中,反应通过加成机理进行,生成 2-oxo-1a,2,3,7b-tetrahydrooxireno[2,3- c ]quinoline-7b-carbonitriles,从中生成 4-hydroxy-3-methoxy-随后通过用甲醇打开环氧化物环形成2-氧代-1,2,3,4-四氢喹啉-4-腈。这些反应的一些次要产物也被分离出来。1 H、13 C和15测量制备的化合物的 N NMR 光谱,并使用适当的二维光谱分配所有共振。
更新日期:2021-06-15
中文翻译:

叔 2-氯酮与氰离子的反应:应用于 3-氯喹啉二酮
由于 C-3 原子上取代基的强位阻,3-Chloroquinoline-2,4-diones 与二甲基甲酰胺中的氰离子反应生成 3-cyanoquinoline-2,4-diones,产率很小。如果该位置的取代基是甲基,则可以获得良好的收率。在甲醇溶液中,反应通过加成机理进行,生成 2-oxo-1a,2,3,7b-tetrahydrooxireno[2,3- c ]quinoline-7b-carbonitriles,从中生成 4-hydroxy-3-methoxy-随后通过用甲醇打开环氧化物环形成2-氧代-1,2,3,4-四氢喹啉-4-腈。这些反应的一些次要产物也被分离出来。1 H、13 C和15测量制备的化合物的 N NMR 光谱,并使用适当的二维光谱分配所有共振。


























 京公网安备 11010802027423号
京公网安备 11010802027423号