Cell Calcium ( IF 4 ) Pub Date : 2021-06-12 , DOI: 10.1016/j.ceca.2021.102435 Maxime Guéguinou 1 , Romain Felix 2 , Séverine Marionneau-Lambot 3 , Thibauld Oullier 4 , Aubin Penna 5 , Sana Kouba 2 , Audrey Gambade 2 , Yann Fourbon 2 , David Ternant 6 , Christophe Arnoult 6 , Gaëlle Simon 7 , Ana Maria Bouchet 2 , Aurélie Chantôme 2 , Thomas Harnois 5 , Jean-Pierre Haelters 7 , Paul-Alain Jaffrès 7 , Gunther Weber 2 , Philippe Bougnoux 2 , François Carreaux 8 , Olivier Mignen 9 , Christophe Vandier 2 , Marie Potier-Cartereau 2
|
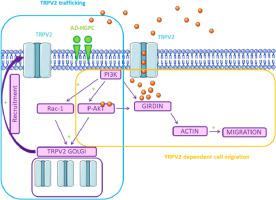
The Transient Receptor Potential Vanilloid type 2 (TRPV2) channel is highly selective for Ca2+ and can be activated by lipids, such as LysoPhosphatidylCholine (LPC). LPC analogues, such as the synthetic alkyl-ether-lipid edelfosine or the endogenous alkyl-ether-lipid Platelet Activating Factor (PAF), modulates ion channels in cancer cells. This opens the way to develop alkyl-ether-lipids for the modulation of TRPV2 in cancer. Here, we investigated the role of 2-Acetamido-2-Deoxy-l-O-Hexadecyl-rac-Glycero-3-PhosphatidylCholine (AD-HGPC), a new alkyl-ether-lipid (LPC analogue), on TRPV2 trafficking and its impact on Ca2+ -dependent cell migration. The effect of AD-HGPC on the TRPV2 channel and tumour process was further investigated using calcium imaging and an in vivo mouse model. Using molecular and pharmacological approaches, we dissected the mechanism implicated in alkyl-ether-lipids sensitive TRPV2 trafficking. We found that TRPV2 promotes constitutive Ca2+ entry, leading to migration of highly metastatic breast cancer cell lines through the PI3K/Akt-Girdin axis. AD-HGPC addresses the functional TRPV2 channel in the plasma membrane through Golgi stimulation and PI3K/Akt/Rac-dependent cytoskeletal reorganization, leading to constitutive Ca2+ entry and breast cancer cell migration (without affecting the development of metastasis), in a mouse model. We describe, for the first time, the biological role of a new alkyl-ether-lipid on TRPV2 channel trafficking in breast cancer cells and highlight the potential modulation of TRPV2 by alkyl-ether-lipids as a novel avenue for research in the treatment of metastatic cancer.
中文翻译:

合成烷基醚脂质通过癌细胞中的 PI3K/Akt-girdin 轴促进 TRPV2 通道运输并增加乳腺肿瘤体积
Transient Receptor Potential Vanilloid type 2 (TRPV2) 通道对 Ca 2+具有高度选择性,可以被脂质激活,例如溶血磷脂酰胆碱 (LPC)。LPC 类似物,例如合成的烷基醚脂质依地福辛或内源性烷基醚脂质血小板激活因子 (PAF),可调节癌细胞中的离子通道。这为开发用于调节癌症中的 TRPV2 的烷基醚脂质开辟了道路。在这里,我们研究了 2-Acetamido-2-Deoxy-10-Hexadecyl-rac-Glycero-3-PhosphatidylCholine (AD-HGPC),一种新的烷基醚脂质(LPC 类似物)对 TRPV2 贩运的作用及其影响Ca 2+依赖性细胞迁移。使用钙成像和体内实验进一步研究了 AD-HGPC 对 TRPV2 通道和肿瘤过程的影响老鼠模型。使用分子和药理学方法,我们剖析了与烷基醚脂质敏感的 TRPV2 贩运有关的机制。我们发现 TRPV2 促进组成型 Ca 2+进入,导致高度转移的乳腺癌细胞系通过 PI3K/Akt-Girdin 轴迁移。AD-HGPC 通过高尔基体刺激和 PI3K/Akt/Rac 依赖性细胞骨架重组处理质膜中的功能性 TRPV2 通道,导致组成型 Ca 2+在小鼠模型中进入和乳腺癌细胞迁移(不影响转移的发展)。我们首次描述了一种新的烷基醚脂质对乳腺癌细胞中 TRPV2 通道运输的生物学作用,并强调了烷基醚脂质对 TRPV2 的潜在调节,作为研究治疗乳腺癌的新途径转移性癌症。


























 京公网安备 11010802027423号
京公网安备 11010802027423号