Advances in Colloid and Interface Science ( IF 15.6 ) Pub Date : 2021-06-11 , DOI: 10.1016/j.cis.2021.102433 Jarl B Rosenholm 1
|
The elegance and general applicability of classical thermodynamics made a great impression on Albert Einstein as quoted:
A theory is the more impressive the greater the simplicity of its premises, the more different kinds of things it relates and the more extended its area of applicability. Therefore the deep impression that classical thermodynamics made upon me. It is the only physical theory of universal content, which I am convinced will never be overthrown, within the framework of applicability of its basic concepts.
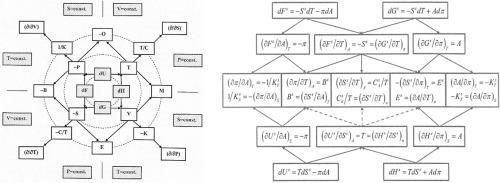
In this review, basic relationships between partial derivatives of internal energy, enthalpy, Helmholtz and Gibbs (free) energies are presented in a condensed and self-consistent “Thermodynamic Wheel of Connections” (TWC). As a support for experimentalists a complete set of first- and second-order partial derivatives of basic state functions (U, F, H, G) derived with respect to state variables (P, T, V, S) under isothermal, isobaric, isochoric and isentropic conditions are presented as a Table. The basic TWC-network remains unchanged when expanded by additional conjugative state parameter pairs, such as chemical potential – amount of substance and surface/interfacial tension – contact area. The extension enables characterization of first- and second-order phase transitions of bulk phases and interphases in terms of first-, second- and third-order partial derivatives of Gibbs energy as well as by first- and second-order partial derivatives of chemical potential and Derjaguin's disjoining pressure. Semi-three-dimensional interface (Guggenheim convention) state functions are derived by subtracting corresponding parameters from total state functions. Then properties become dependent on the location and extension of the interface. For truly two-dimensional mono-molecular Langmuir films (Gibbs convention), first- and second-order partial derivatives of basic interfacial state functions (Us, Fs, Hs, Gs) may be derived with respect to state variables (π, T, A, Ss) under isothermal, isobaric, isoareal and isentropic conditions. They are assembled as interfacial “Thermodynamic Family Three” (TFT) s. Replacing π by P, A by V and omitting upper index s it is converted to previously published TFT for bulk phases.
中文翻译:

基本平衡热力学的合理方法
经典热力学的优雅和普遍适用性给阿尔伯特·爱因斯坦留下了深刻的印象,引述如下:
一个理论的前提越简单,它所涉及的事物种类就越多,它的适用范围就越广,就越令人印象深刻。因此经典热力学给我留下了深刻的印象。它是唯一关于普遍内容的物理理论,我相信它在其基本概念的适用性框架内永远不会被推翻。
在这篇综述中,内能、焓、亥姆霍兹和吉布斯(自由)能的偏导数之间的基本关系以压缩和自洽的“热力学连接轮”(TWC)形式呈现。作为对实验者的支持,关于状态变量(P、T、V、S)导出的基本状态函数(U、F、H、G)的一阶和二阶偏导数的完整集合) 在等温、等压、等容和等熵条件下如表所示。当通过额外的共轭状态参数对扩展时,基本的 TWC 网络保持不变,例如化学势 - 物质和表面/界面张力 - 接触面积。该扩展能够根据吉布斯能的一阶、二阶和三阶偏导数以及化学势的一阶和二阶偏导数来表征体相和中间相的一阶和二阶相变以及 Derjaguin 的分离压力。半三维界面(古根海姆约定)状态函数是通过从总状态函数中减去相应参数得出的。然后属性变得依赖于接口的位置和扩展。U s , F s , H s , G s ) 可以在等温、等压、等面积和等熵条件下相对于状态变量 ( π, T, A, S s ) 导出。它们组装成界面“热力学家庭三”(TFT)š。用P代替π,用V代替A并省略上指数s,它被转换为先前公布的体相 TFT。


























 京公网安备 11010802027423号
京公网安备 11010802027423号