当前位置:
X-MOL 学术
›
J. Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Longer charged amino acids favor β-strand formation in hairpin peptides
Journal of Peptide Science ( IF 2.1 ) Pub Date : 2021-06-10 , DOI: 10.1002/psc.3333 Jing-Yuan Chang, Nian-Zhi Li, Wei-Ming Wang, Chih-Ting Liu, Chen-Hsu Yu, Yan-Chen Chen, Daniel Lu, Pei-Hsuan Lin, Cheng-Hsin Huang, Orika Kono, Tzu-Yi Yang, Yi-Ting Sun, Pei-Yu Huang, Yen-Jin Pan, Ting-Hsuan Chen, Mu-Chun Liu, Shou-Ling Huang, Shing-Jong Huang, Richard P. Cheng
Journal of Peptide Science ( IF 2.1 ) Pub Date : 2021-06-10 , DOI: 10.1002/psc.3333 Jing-Yuan Chang, Nian-Zhi Li, Wei-Ming Wang, Chih-Ting Liu, Chen-Hsu Yu, Yan-Chen Chen, Daniel Lu, Pei-Hsuan Lin, Cheng-Hsin Huang, Orika Kono, Tzu-Yi Yang, Yi-Ting Sun, Pei-Yu Huang, Yen-Jin Pan, Ting-Hsuan Chen, Mu-Chun Liu, Shou-Ling Huang, Shing-Jong Huang, Richard P. Cheng
|
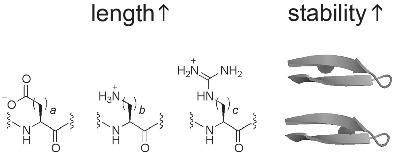
Interactions between charged amino acids significantly influence the structure and function of proteins. The encoded charged amino acids Asp, Glu, Arg, and Lys have different number of hydrophobic methylenes linking the backbone to the charged functionality. It remains to be fully understood how does this difference in the number of methylenes affect protein structure stability. Protein secondary structures are the fundamental three-dimensional building blocks of protein structures. β-Sheet structures are particularly interesting, because these structures have been associated with a number of protein misfolding diseases. Herein, we report the effect of charged amino acid side chain length at two β-strand positions individually on the stability of a β-hairpin. The charged amino acids include side chains with a carboxylate, an ammonium, or a guanidinium group. The experimental peptides, fully folded reference peptides, and fully unfolded reference peptides were synthesized by solid phase peptide synthesis and analyzed by 2D NMR methods including TOCSY, DQF-COSY, and ROESY. Sequence specific assignments were performed for all peptides. The chemical shift data were used to derive the fraction folded population and the folding free energy for the experimental peptides. Results showed that the fraction folded population increased with increasing charged amino acid side chain length. These results should be useful for developing functional peptides that adopt the β-conformation.
中文翻译:

较长的带电氨基酸有利于发夹肽中 β 链的形成
带电氨基酸之间的相互作用显着影响蛋白质的结构和功能。编码的带电氨基酸 Asp、Glu、Arg 和 Lys 具有不同数量的疏水亚甲基,将主链连接到带电官能团。亚甲基数量的这种差异如何影响蛋白质结构的稳定性仍有待充分了解。蛋白质二级结构是蛋白质结构的基本三维构建块。β-Sheet 结构特别有趣,因为这些结构与许多蛋白质错误折叠疾病有关。在此,我们报告了两个β-链位置的带电氨基酸侧链长度分别对β-发夹稳定性的影响。带电荷的氨基酸包括带有羧酸盐、铵盐、或胍基团。通过固相肽合成法合成实验肽、完全折叠的参考肽和完全展开的参考肽,并通过包括TOCSY、DQF-COSY和ROESY的2D NMR方法进行分析。对所有肽进行序列特异性分配。化学位移数据用于推导实验肽的折叠群体分数和折叠自由能。结果表明,随着带电氨基酸侧链长度的增加,部分折叠群体增加。这些结果对于开发采用β构象的功能性肽应该是有用的。DQF-COSY 和 ROESY。对所有肽进行序列特异性分配。化学位移数据用于推导实验肽的折叠群体分数和折叠自由能。结果表明,随着带电氨基酸侧链长度的增加,部分折叠群体增加。这些结果对于开发采用β构象的功能性肽应该是有用的。DQF-COSY 和 ROESY。对所有肽进行序列特异性分配。化学位移数据用于推导实验肽的折叠群体分数和折叠自由能。结果表明,随着带电氨基酸侧链长度的增加,部分折叠群体增加。这些结果对于开发采用β构象的功能性肽应该是有用的。
更新日期:2021-08-07
中文翻译:

较长的带电氨基酸有利于发夹肽中 β 链的形成
带电氨基酸之间的相互作用显着影响蛋白质的结构和功能。编码的带电氨基酸 Asp、Glu、Arg 和 Lys 具有不同数量的疏水亚甲基,将主链连接到带电官能团。亚甲基数量的这种差异如何影响蛋白质结构的稳定性仍有待充分了解。蛋白质二级结构是蛋白质结构的基本三维构建块。β-Sheet 结构特别有趣,因为这些结构与许多蛋白质错误折叠疾病有关。在此,我们报告了两个β-链位置的带电氨基酸侧链长度分别对β-发夹稳定性的影响。带电荷的氨基酸包括带有羧酸盐、铵盐、或胍基团。通过固相肽合成法合成实验肽、完全折叠的参考肽和完全展开的参考肽,并通过包括TOCSY、DQF-COSY和ROESY的2D NMR方法进行分析。对所有肽进行序列特异性分配。化学位移数据用于推导实验肽的折叠群体分数和折叠自由能。结果表明,随着带电氨基酸侧链长度的增加,部分折叠群体增加。这些结果对于开发采用β构象的功能性肽应该是有用的。DQF-COSY 和 ROESY。对所有肽进行序列特异性分配。化学位移数据用于推导实验肽的折叠群体分数和折叠自由能。结果表明,随着带电氨基酸侧链长度的增加,部分折叠群体增加。这些结果对于开发采用β构象的功能性肽应该是有用的。DQF-COSY 和 ROESY。对所有肽进行序列特异性分配。化学位移数据用于推导实验肽的折叠群体分数和折叠自由能。结果表明,随着带电氨基酸侧链长度的增加,部分折叠群体增加。这些结果对于开发采用β构象的功能性肽应该是有用的。


























 京公网安备 11010802027423号
京公网安备 11010802027423号