Environmental Toxicology and Pharmacology ( IF 4.3 ) Pub Date : 2021-06-10 , DOI: 10.1016/j.etap.2021.103688 Elizabeth Goya-Jorge 1 , Mazia Amber 2 , Rafael Gozalbes 3 , Lisa Connolly 2 , Stephen J Barigye 3
|
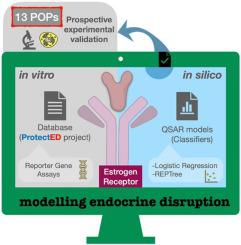
Multiple substances are considered endocrine disrupting chemicals (EDCs). However, there is a significant gap in the early prioritization of EDC’s effects. In this work, in silico and in vitro methods were used to model estrogenicity. Two Quantitative Structure-Activity Relationship (QSAR) models based on Logistic Regression and REPTree algorithms were built using a large and diverse database of estrogen receptor (ESR) agonism. A 10-fold external validation demonstrated their robustness and predictive capacity. Mechanistic interpretations of the molecular descriptors (C-026, nArOH,PW5, B06[Br-Br]) used for modelling suggested that the heteroatomic fragments, aromatic hydroxyls, and bromines, and the relative bond accessibility areas of molecules, are structural determinants in estrogenicity. As validation of the QSARs, ESR transactivity of thirteen persistent organic pollutants (POPs) and suspected EDCs was tested in vitro using the MMV-Luc cell line. A good correspondence between predictions and experimental bioassays demonstrated the value of the QSARs for prioritization of ESR agonist compounds.
中文翻译:

使用计算机模拟和体外方法评估化学诱导的雌激素性
多种物质被认为是内分泌干扰物 (EDC)。然而,EDC 影响的早期优先排序存在显着差距。在这项工作中,计算机和体外方法被用来模拟雌激素。使用大量多样的雌激素受体 (ESR) 激动数据库建立了两个基于逻辑回归和 REPTree 算法的定量构效关系 (QSAR) 模型。10 倍的外部验证证明了它们的稳健性和预测能力。用于建模的分子描述符(C-026、nArOH、PW5、B06[Br-Br])的机械解释表明,杂原子片段、芳香羟基和溴以及分子的相对键可及区域是结构决定因素雌激素。作为 QSAR 的验证,在体外测试了 13 种持久性有机污染物 (POP) 和疑似 EDC 的 ESR 交易性使用 MMV-Luc 细胞系。预测与实验生物测定之间的良好对应证明了 QSAR 对 ESR 激动剂化合物优先排序的价值。



























 京公网安备 11010802027423号
京公网安备 11010802027423号